
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
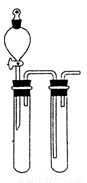
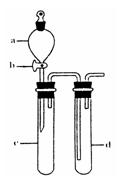
(1)ij��ȤС�������ͼ��ʾװ����ȡSO2

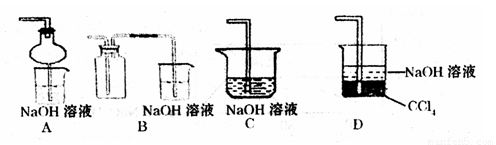
������ʵ�鷽��������ͼ��ʾװ����ȡ����SO2���Լ���_______(����ţ���
A��Na2SO3��Һ��ϡ����
B��Na2SO3������Ũ����
C�������������
D��ͭ��Ũ����
��a������������_______��
��β������װ�õ�����˳����b��( )�� ( )��e��
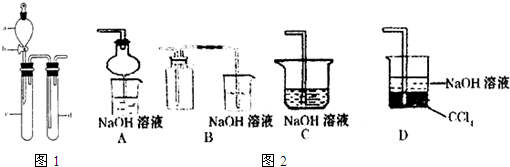
(2)Ϊ�˻�������SO2�������о���Ա���������õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)���ո��±��պ������������SO2�������Ʊ������̾���( �������̣�������ʾ��ͼ���£�
�������̣�������ʾ��ͼ���£�

��֪������Һ��pH<2�����еĽ���������Ҫ��Mn2+��������������Fe2����Al3���������������ӡ��йؽ��������γ������������ʱ��Һ��pH���±���

��ش�
�ٺ�Al3�����γ�������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��________________________��
�ڽ�����������Ҫ��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
�����������м���MnO2�۵�Ŀ����______________________________________________;
��Ӧ�����ӷ���ʽ��_________________________________________________________��
����ʯ�ҽ�����pH��pHӦ���ڵķ�Χ��___________________________________��
����������Ҫ�ɷ���____________________________________��
��1����B (2��)
�ڷ�Һ©�� (2��)
��d c (2��)
��2����Al3++3H2O  Al(OH)3+3H +
(2��)
Al(OH)3+3H +
(2��)
��SO2��MnO2��MnSO4 (2��)
�۽�Fe2������ΪFe3�� (2��)
2Fe2����MnO2��4H����2Fe3����Mn2����2H2O (2��)
��4.7��pH��8.3 (2��)
��������������������������� ��2�֣�
����������1���ٸ�װ�����ڹ�Һ��ҺҺ��ϲ�������װ��
A��Na2SO3��Һ��HNO3��Ӧʱ������������������Ʊ�������ԭΪһ����������A�����ϣ�
B��Na2SO3������Ũ������ȷ�Ӧ������Ũ�����ѻӷ��Կ��Է�Ӧ���ɶ����������壬��B���ϣ�
C����ȼ�ղ���Ҫ��Һ©������C�����ϣ�
D��ͭ��Ũ���ᷴӦ��Ҫ���ȣ���D�����ϣ�
��ѡB��
��a�����������ǣ���Һ©�����𰸣���Һ©����
����ͼ��β������װ�õ�����˳����b��(d)�� (c)��e���𰸣�d c ��
��2���ٺ�Al3�����γ�������ˮ����������Al3�� ˮ�������Al(OH)3 ���壬���������ԣ�����ˮ�е����������ʣ����ӷ���ʽΪ��Al3++3H2O
 Al(OH)3+3H + ���𰸣�Al3++3H2O
Al(OH)3+3H + ���𰸣�Al3++3H2O  Al(OH)3+3H + �� �ڵ�Ʒλ���̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2������MnO2��SO2����������ԭ��Ӧ�Ļ�ѧ����ʽΪSO2+MnO2=MnSO4��
Al(OH)3+3H + �� �ڵ�Ʒλ���̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2������MnO2��SO2����������ԭ��Ӧ�Ļ�ѧ����ʽΪSO2+MnO2=MnSO4��
�ʴ�Ϊ��SO2��MnO2��MnSO4 ��������������ֻ��Fe2�����л�ԭ�ԣ����Ա�MnO2������������������Fe3������Ӧ�����ӷ���ʽΪ2Fe2��+MnO2+4H��=2Fe3��+Mn2��+2H2O��
�ʴ�Ϊ����Fe2������ΪFe3�� 2Fe2����MnO2��4H����2Fe3����Mn2����2H2O ������Һ�м���ʯ�ҽ�������pH���������dz�ȥ�����к���Fe3����Al3�������ӣ���ͼ���Կ���������4.7���Խ�Fe3����Al3����ȥ��С��8.3�Ƿ�ֹMn2��Ҳ����������ֻҪ����pHֵ��4.7��8.3�伴�ɣ�
�ʴ�Ϊ��4.7��pH��8.3 ����Fe3����Al3��������ͨ����pHֵ��ת��Ϊ������������������������ͬʱ�����ܵ�����ƣ�����������Ҫ��������������������������ƣ�
�ʴ�Ϊ���������������������������
���㣺�����������Ⱦ�����������������Ʊ�ԭ����װ��ѡ��ҵ��������SO2����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��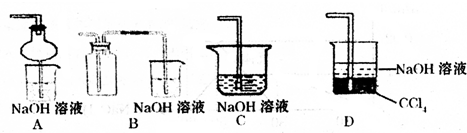
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
��1�������й������˵����ȷ���� ������ţ���
A. SO2��NO2��CO2���ᵼ��������γ�
B. NO������ˮ�����Բ������������Ⱦ
C. ȼúʱ�����������������Լ�������IJ���
D. ���������Դ�����Լ�������IJ���
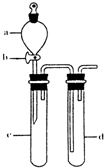
��2��ij��ȤС�������ͼװ����ȡ��̽��SO2��������ʡ�
������ʵ�鷽������������ͼ��ʾװ����ȡ����SO2���� ������ţ���
A. Na2SO3��Һ��HNO3 B. Na2SO3������Ũ����
C. �������ڴ�����ȼ�� D.ͭ��ŨH2SO4
����װ���������װ�������Եķ����ǣ��رջ���b��
��ָ����ʦָ��Ӧ����һβ������װ�ã�������ͬѧ�����������װ�ã����к�������
������ţ���
��С��ͬѧ������Թ�d�м���FeCl3��Һ����֤SO2�Ļ�ԭ�ԡ�Ϊ����֤SO2��Fe3+������������ԭ��Ӧ��������ͨ������SO2��ȡ�Թ�d�е���Һ���ֳ����ݣ������������ʵ�飺
����A������һ����Һ�м���KmnO4��Һ���Ϻ�ɫ��ȥ
����B�����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ���
����C������������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ�����и�����ѧ�����п��Ի�ѧ���� ���ͣ�ʵ����
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
��1�������й������˵����ȷ���� ������ţ���
A. SO2��NO2��CO2���ᵼ��������γ�
B. NO������ˮ�����Բ������������Ⱦ
C. ȼúʱ�����������������Լ�������IJ���
D. ���������Դ�����Լ�������IJ���
��2��ij��ȤС�������ͼװ����ȡ��̽��SO2��������ʡ�
������ʵ�鷽������������ͼ��ʾװ����ȡ����SO2���� ������ţ���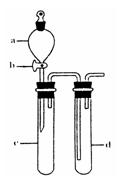
A. Na2SO3��Һ��HNO3 B. Na2SO3������Ũ����
C. �������ڴ�����ȼ�� D.ͭ��ŨH2SO4
����װ���������װ�������Եķ����ǣ��رջ���b��
��ָ����ʦָ��Ӧ����һβ������װ�ã�������ͬѧ�����������װ�ã����к�������
������ţ���
��С��ͬѧ������Թ�d�м���FeCl3��Һ����֤SO2�Ļ�ԭ�ԡ�Ϊ����֤SO2��Fe3+������������ԭ��Ӧ��������ͨ������SO2��ȡ�Թ�d�е���Һ���ֳ����ݣ������������ʵ�飺
����A������һ����Һ�м���KmnO4��Һ���Ϻ�ɫ��ȥ
����B�����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ���
����C������������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡ�����и�����ѧ�����п��Ի�ѧ���� ���ͣ�ʵ����
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
��1�������й������˵����ȷ���� ������ţ���
A. SO2��NO2��CO2���ᵼ��������γ�
B. NO������ˮ�����Բ������������Ⱦ
C. ȼúʱ�����������������Լ�������IJ���
D. ���������Դ�����Լ�������IJ���
��2��ij��ȤС�������ͼװ����ȡ��̽��SO2��������ʡ�
������ʵ�鷽������������ͼ��ʾװ����ȡ����SO2���� ������ţ���
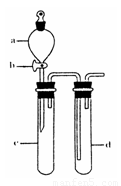
A. Na2SO3��Һ��HNO3 B. Na2SO3������Ũ����
C. �������ڴ�����ȼ�� D.ͭ��ŨH2SO4
����װ���������װ�������Եķ����ǣ��رջ���b��
��ָ����ʦָ��Ӧ����һβ������װ�ã�������ͬѧ�����������װ�ã����к�������
������ţ���
��С��ͬѧ������Թ�d�м���FeCl3��Һ����֤SO2�Ļ�ԭ�ԡ�Ϊ����֤SO2��Fe3+������������ԭ��Ӧ��������ͨ������SO2��ȡ�Թ�d�е���Һ���ֳ����ݣ������������ʵ�飺
����A������һ����Һ�м���KmnO4��Һ���Ϻ�ɫ��ȥ
����B�����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ���
����C������������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ԭ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com