
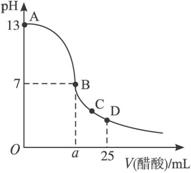
��1��д������������Һ�������Һ��Ӧ�����ӷ���ʽ��__________________________��
��2��������������Һ�����ʵ���Ũ��Ϊ_____________mol��L-1��
��3����B�㣬a_____________12.5 mL������ڡ���С�ڡ����ڡ�����ͬ�������������ȵ��������ƺʹ�����Һ��϶���ǡ�ó����ԣ�����ǰc(NaOH)____________c(CH3COOH)�����ǰ����c(H+)�ͼ���c(OH-)�Ĺ�ϵ��c(H+)_____________c(OH-)��
��4����D�㣬��Һ������Ũ�ȴ�С��ϵΪ_____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������38�ס�2008ȫ����ʡ�и߿�ģ��������(��ٰ�)������ѧ ��ٰ� ���ͣ�022
��25 mL����������Һ����μ���0.2 mol/L������Һ���ζ���������ͼ��ʾ��
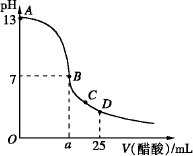
(1)д������������Һ�������Һ��Ӧ�����ӷ���ʽ________��
(2)������������Һ�����ʵ���Ũ��Ϊ________mol��L��1
(3)��B�㣬a________12.5 mL(����ڡ���С�ڡ����ڡ�����ͬ)�����������ȵ��������ƺʹ�����Һ��϶���ǡ�ó����ԣ�����ǰc(NaOH)________c(CH3COOH)�����ǰ����c(H+)�ͼ���c(OH��)�Ĺ�ϵ��c(H+)________c(OH��)��
(4)��D�㣬��Һ������Ũ�ȴ�С��ϵΪ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ���ƽ�ص�һ��ѧ�߶��ڶ��ε��п������ƻ�ѧ���⣨�������� ���ͣ������
��ÿ��2�֣���10�֣���1���ڴ���CuSO4��5H2O�����г���������Fe2+�����ᴿʱΪ�˳�ȥFe2+�������������������ʹFe2+����ΪFe3+���������ʿɲ��õ���_____
A��KMnO4 �� B�� H2O2 �� C�� Cl2ˮ ���� D�� HNO3
Ȼ���ټ��������ʵ����ʵ�������ҺpH=4��ʹFe3+ת��ΪFe(OH)3�����Դﵽ��ȥFe3+
������ʧCuSO4��Ŀ�ģ�������ҺpH��ѡ�������е�________
A. NaOH B. NH3��H2O C. CuO D. Cu(OH)2
��2����25 mL����������Һ����μ���0.2mol/L������Һ���õ���������ͼ��ʾ 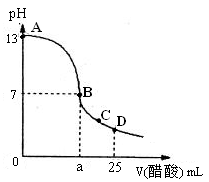
�١�д������������Һ�������Һ��Ӧ�����ӷ���ʽ
�ڡ�������������Һ�����ʵ���Ũ��Ϊ
�ۡ���B�㣬a 12.5 mL������ڡ�����С�ڡ����ڡ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ���ƽ�ص�һ��ѧ�߶��ڶ��ε��п������ƻ�ѧ���⣨�����棩 ���ͣ������
��ÿ��2�֣���10�֣���1���ڴ���CuSO4��5H2O�����г���������Fe2+�����ᴿʱΪ�˳�ȥFe2+�������������������ʹFe2+����ΪFe3+���������ʿɲ��õ���_____
A��KMnO4 �� B�� H2O2 �� C�� Cl2 ˮ ���� D�� HNO3
Ȼ���ټ��������ʵ����ʵ�������ҺpH=4��ʹFe3+ת��ΪFe(OH)3�����Դﵽ��ȥFe3+
������ʧCuSO4��Ŀ�ģ�������ҺpH��ѡ�������е�________
A. NaOH B. NH3��H2O C. CuO D. Cu(OH)2
��2����25 mL����������Һ����μ���0.2mol/L������Һ���õ���������ͼ��ʾ
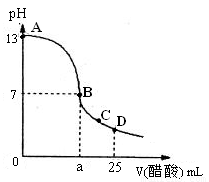
�١�д������������Һ�������Һ��Ӧ�����ӷ���ʽ
�ڡ�������������Һ�����ʵ���Ũ��Ϊ
�ۡ���B�㣬a 12.5 mL������ڡ�����С�ڡ����ڡ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com