
��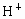 ��
��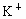 ��
��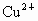 ��
��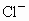 ��
��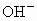 ��
��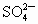 �������У�ѡ���ʵ���������ɷ�����������ĵ���ʣ����е��(������ֻ��ʹ��һ��)��
�������У�ѡ���ʵ���������ɷ�����������ĵ���ʣ����е��(������ֻ��ʹ��һ��)��
(1)��̼��Ϊ�缫���е�⣬����ʵĺ������٣�ˮ�����ֲ��䣬���������������ɣ����������ͬ����õ���ʵĻ�ѧʽΪ__________�����ĵ缫��Ӧ_________�������ܷ���ʽ��____________��
(2)�Բ�˿Ϊ�缫���е�⣬ˮ�����٣�����ʵĺ������ֲ��䣬���������������ɣ����������Ϊ2��1����õ���ʵĻ�ѧʽΪ____________���缫��ӦʽΪ_____________��
(3)�ö��Ե缫���е�⣬����ʵĺ������٣�ˮ�ĺ���Ҳ���٣�pH�½��������ʵĻ�ѧʽΪ_________�������ܷ���ʽ��_________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058
��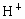 ��
�� ��
��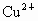 ��
��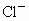 ��
��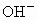 ��
��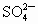 �������У�ѡ���ʵ���������ɷ�����������ĵ���ʣ����е��(������ֻ��ʹ��һ��)��
�������У�ѡ���ʵ���������ɷ�����������ĵ���ʣ����е��(������ֻ��ʹ��һ��)��
(1)��̼��Ϊ�缫���е�⣬����ʵĺ������٣�ˮ�����ֲ��䣬���������������ɣ����������ͬ����õ���ʵĻ�ѧʽΪ__________�����ĵ缫��Ӧ_________�������ܷ���ʽ��____________��
(2)�Բ�˿Ϊ�缫���е�⣬ˮ�����٣�����ʵĺ������ֲ��䣬���������������ɣ����������Ϊ2��1����õ���ʵĻ�ѧʽΪ____________���缫��ӦʽΪ_____________��
(3)�ö��Ե缫���е�⣬����ʵĺ������٣�ˮ�ĺ���Ҳ���٣�pH�½��������ʵĻ�ѧʽΪ_________�������ܷ���ʽ��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ��ʡ�߶�12���¿���ѧ�Ծ� ���ͣ������
��NO3-��SO42-��H+��Cu2+��Ba2+��Ag+��Cl-��������ѡ���ʵ���������ɵ���ʣ����ö��Ե缫������Һ���е�⡣�������ֱ�ų����壬�������Ϊ1��1������ʵĻ�ѧʽ������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com