
(12��)��������Ϊԭ�������������õ����Է�ˮ����Ԫ�غ������ߣ�Ϊ��������ŷţ����û�ѧ���������������ˮ������������±���
��1�����������ε�Ksp ��2��������Ⱦ���ŷ�Ũ�ȼ������ŷű�
�ش��������⣺
�Ÿ����Ṥ���ŷŵķ�ˮ����������ʵ���Ũ��c(H2SO4)��______________mol��L��1��
��д��������Ca3(AsO4)2��Ksp����ʽ��Ksp[Ca3(AsO4)2]��______________���������Һ
��Al3����Fe3����Ũ�Ⱦ�Ϊ1.0��10��4mol��L��1��c(AsO43��)�������_______________mol��L��1��
�ǹ����ŷų������Է�ˮ�е������飨H3AsO3���ᣩ���׳�������Ͷ��MnO2�Ƚ�������������飨H3AsO4���ᣩ��д���÷�Ӧ�����ӷ���ʽ_________________________��
���ڴ��������ˮʱ���÷ֶ�ʽ�������ˮ��Ͷ����ʯ�ҵ���pH��2����Ͷ����ʯ�ҽ�pH���ڵ�8����ʹ�������Ca3(AsO4)2��ʽ������
�ٽ�pH���ڵ�2ʱ��ˮ���д�������������������Ҫ�ɷֵĻ�ѧʽΪ______________��
��Ca3(AsO4)2��pH���ڵ�8���Ҳſ�ʼ������ԭ��Ϊ_____________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)��������Ϊԭ�������������õ����Է�ˮ����Ԫ�غ������ߣ�Ϊ��������ŷţ����û�ѧ���������������ˮ������������±���
| ������ | Ksp |
| Ca3(AsO4)2 | 6.8��10��19 |
| AlAsO4 | 1.6��10��16 |
| FeAsO4 | 5.7��10��21 |
��1�����������ε�Ksp
| ��Ⱦ�� | H2SO4 | As |
| Ũ�� | 28.42 g/L | 1.6 g��L��1 |
| �ŷű� | pH 6��9 | 0.5 mg��L��1 |
��2��������Ⱦ���ŷ�Ũ�ȼ������ŷű�
�ش��������⣺
�Ÿ����Ṥ���ŷŵķ�ˮ����������ʵ���Ũ��c(H2SO4)��______________mol��L��1��
��д��������Ca3(AsO4)2��Ksp����ʽ��Ksp[Ca3(AsO4)2]��______________���������Һ
��Al3����Fe3����Ũ�Ⱦ�Ϊ1.0��10��4mol��L��1��c(AsO43��)�������_______________mol��L��1��
�ǹ����ŷų������Է�ˮ�е������飨H3AsO3���ᣩ���׳�������Ͷ��MnO2�Ƚ�������
������飨H3AsO4���ᣩ��д���÷�Ӧ�����ӷ���ʽ________________________________��
���ڴ��������ˮʱ���÷ֶ�ʽ�������ˮ��Ͷ����ʯ�ҵ���pH��2����Ͷ����ʯ�ҽ�pH
���ڵ�8����ʹ�������Ca3(AsO4)2��ʽ������
�ٽ�pH���ڵ�2ʱ��ˮ���д�������������������Ҫ�ɷֵĻ�ѧʽΪ______________��
��Ca3(AsO4)2��pH���ڵ�8���Ҳſ�ʼ������ԭ��Ϊ_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ������Զ��ѧ����ʵ����һ��ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ������
(12��)��������Ϊԭ�������������õ����Է�ˮ����Ԫ�غ������ߣ�Ϊ��������ŷţ����û�ѧ���������������ˮ������������±���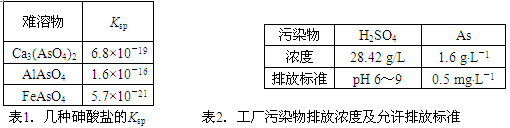
�ش��������⣺
�Ÿ����Ṥ���ŷŵķ�ˮ����������ʵ���Ũ��c(H2SO4)�� ________mol��L��1��
��д��������Ca3(AsO4)2��Ksp����ʽ��Ksp[Ca3(AsO4)2]�� ________���������Һ��Al3����Fe3����Ũ�Ⱦ�Ϊ1.0��10��4mol��L��1��c(AsO43��)������� ___mol��L��1��
�ǹ����ŷų������Է�ˮ�е������飨H3AsO3���ᣩ���׳�������Ͷ��MnO2�Ƚ�������������飨H3AsO4���ᣩ��д���÷�Ӧ�����ӷ���ʽ ��
���ڴ��������ˮʱ���÷ֶ�ʽ�������ˮ��Ͷ����ʯ�ҵ���pH��2����Ͷ����ʯ�ҽ�pH���ڵ�8����ʹ�������Ca3(AsO4)2��ʽ������
��pH���ڵ�2ʱ��ˮ���д�������������������Ҫ�ɷֵĻ�ѧʽΪ __��
��Ca3(AsO4)2��pH���ڵ�8���Ҳſ�ʼ������ԭ��Ϊ _____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ��������ᡢ�����ѧ�����ڶ��ε���������ѧ�Ծ� ���ͣ������
(12��)��������Ϊԭ�������������õ����Է�ˮ����Ԫ�غ������ߣ�Ϊ��������ŷţ����û�ѧ���������������ˮ������������±���
| ������ | Ksp |
| Ca3(AsO4)2 | 6.8��10��19 |
| AlAsO4 | 1.6��10��16 |
| FeAsO4 | 5.7��10��21 |
| ��Ⱦ�� | H2SO4 | As |
| Ũ�� | 28.42 g/L | 1.6 g��L��1 |
| �ŷű� | pH 6��9 | 0.5 mg��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ����ʵ����һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
(12��)��������Ϊԭ�������������õ����Է�ˮ����Ԫ�غ������ߣ�Ϊ��������ŷţ����û�ѧ���������������ˮ������������±���
 �ش��������⣺
�ش��������⣺
�Ÿ����Ṥ���ŷŵķ�ˮ����������ʵ���Ũ��c(H2SO4)�� ________mol��L��1��
��д��������Ca3(AsO4)2��Ksp����ʽ��Ksp[Ca3(AsO4)2]�� ________���������Һ��Al3����Fe3����Ũ�Ⱦ�Ϊ1.0��10��4mol��L��1��c(AsO43��)������� ___mol��L��1��
�ǹ����ŷų������Է�ˮ�е������飨H3AsO3���ᣩ���׳�������Ͷ��MnO2�Ƚ�������������飨H3AsO4���ᣩ��д���÷�Ӧ�����ӷ���ʽ ��
���ڴ��������ˮʱ���÷ֶ�ʽ�������ˮ��Ͷ����ʯ�ҵ���pH��2����Ͷ����ʯ�ҽ�pH���ڵ�8����ʹ�������Ca3(AsO4)2��ʽ������
��pH���ڵ�2ʱ��ˮ���д�������������������Ҫ�ɷֵĻ�ѧʽΪ __��
��Ca3(AsO4)2��pH���ڵ�8���Ҳſ�ʼ������ԭ��Ϊ _____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com