

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
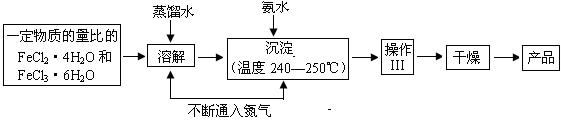
 ČėĘųĢåŠĪ³ÉĪ¢ŠĶĘųÅŻ£¬½«øÕÉś³ÉµÄĖÄŃõ»ÆČżĢśĪ¢Į£°üĪ§£¬Ą“×čÖ¹Ī¢Į£µÄ³¤“ó»ņ¾Ū¼Æ³ÉĶÅ£»¢Ś ”ų ”£
ČėĘųĢåŠĪ³ÉĪ¢ŠĶĘųÅŻ£¬½«øÕÉś³ÉµÄĖÄŃõ»ÆČżĢśĪ¢Į£°üĪ§£¬Ą“×čÖ¹Ī¢Į£µÄ³¤“ó»ņ¾Ū¼Æ³ÉĶÅ£»¢Ś ”ų ”£| ²½Öč | ²Ł×÷ | ĻÖĻ󔢽įĀŪ |
| 1 | ”ų |  |
| 2 | ȔɣĮæ²śĘ·ÓŚŹŌ¹ÜÖŠ¼ÓŹŹĮæ²½Öč1“¦ĄķŗĆČÜŅŗČܽā£¬Åä³ÉČÜŅŗ | ¹ĢĢåČܽā£¬ČÜŅŗ³ŹĒ³»ĘÉ« |
| 3 | ȔɣĮæ²½Öč2ÅäŗĆČÜŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼Ó¼øµĪ20%ĮņĒč»Æ¼ŲČÜŅŗ£¬Õńµ“ | ”ų £¬²śĘ·ŗ¬ÓŠFe3+ |
| 4 | ”ų | ”ų £¬²śĘ·ŗ¬ÓŠFe2+ |
 µĪ¶Ø£¬ÖĮÖÕµćŹ±ĻūŗÄKMnO4ČÜŅŗĢå»ż29.80mL”£
µĪ¶Ø£¬ÖĮÖÕµćŹ±ĻūŗÄKMnO4ČÜŅŗĢå»ż29.80mL”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 Cl2”ü£«MnCl2£«2H2OŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
Cl2”ü£«MnCl2£«2H2OŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ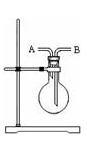
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®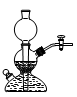 | B£®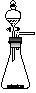 | C£®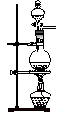 | D£®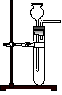 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÅäÖĘ5%NaClČÜŅŗŹ±£¬½«³ĘĮæµÄNaCl·ÅČėÉÕ±ÖŠ¼Ó¼ĘĮæµÄĖ®½Į°čČܽā |
| B£®ÅäÖĘ1mol”¤L£1NaOHČÜŅŗŹ±£¬½«ČܽāŗóµÄNaOHČÜŅŗĮ¢¼“×¢ČėČŻĮæĘæ |
| C£®ÅäÖĘ0£®1mol/LµÄH2SO4ČÜŅŗŹ±£¬½«ĮæČ”µÄÅØH2SO4·ÅČėČŻĮæĘæÖŠ¼ÓĖ®Ļ”ŹĶ |
| D£®·ÖŅŗ²Ł×÷Ź±£¬ĻČ½«·ÖŅŗĀ©¶·ÖŠµÄĻĀ²ćŅŗĢå·Å³ö£¬Č»ŗóŌŁ½«ÉĻ²ćŅŗĢå·Å³ö |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
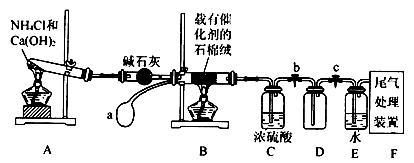
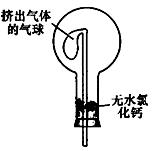
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
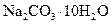 ¾§ĢåÅäÖĘ
¾§ĢåÅäÖĘ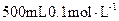 µÄ
µÄ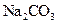 ČÜŅŗ£¬Ēė»Ų“šĻĀĮŠĪŹĢā”£
ČÜŅŗ£¬Ēė»Ų“šĻĀĮŠĪŹĢā”£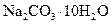 ¾§Ģå_______g”£
¾§Ģå_______g”£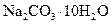 ¾§ĢåŹĒ__________g”£
¾§ĢåŹĒ__________g”£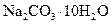 ¾§ĢåŅŃŹ§Č„²æ·Ö½į¾§Ė®”£____________
¾§ĢåŅŃŹ§Č„²æ·Ö½į¾§Ė®”£____________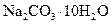 ¾§ĢåÖŠ»ģÓŠÉŁĮæ
¾§ĢåÖŠ»ģÓŠÉŁĮæ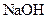 ¹ĢĢ壬ĒŅÓĆĖłÅäÖʵÄ
¹ĢĢ壬ĒŅÓĆĖłÅäÖʵÄ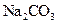 ČÜŅŗÓėŃĪĖį·“Ó¦Ą“²ā¶ØijŃĪĖįµÄĪļÖŹµÄĮæÅØ¶Č£¬Ėł²āŃĪĖįµÄÅØ¶Č»į________”££ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±”¢”°²»±ä”±”££©
ČÜŅŗÓėŃĪĖį·“Ó¦Ą“²ā¶ØijŃĪĖįµÄĪļÖŹµÄĮæÅØ¶Č£¬Ėł²āŃĪĖįµÄÅØ¶Č»į________”££ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±”¢”°²»±ä”±”££©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

| A£®ÉżøßĪĀ¶Č | B£®½µµĶĪĀ¶Č | C£®Ōö“óŃ¹Ēæ | D£®Ōö“óŃ¹Ēæ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com