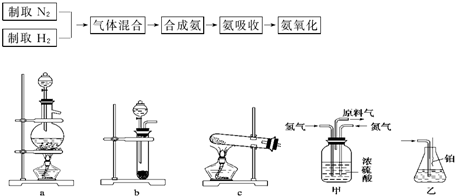
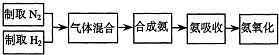
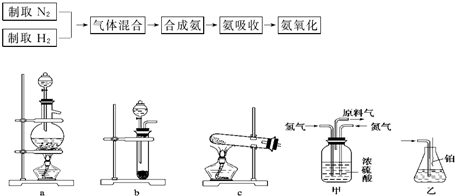
��2009?����һģ��ʵ����ģ��ϳɰ��Ͱ����������������£�

��l����֪ʵ���ҿ��ñ����������ƣ�NaNO
2����Һ�뱥���Ȼ�Ǯ��Һ�����Ⱥ�Ӧ��ȡ������д���÷�Ӧ�Ļ�ѧ����ʽ��
NH
4Cl+NaNO
2NaCl+N
2��+2H
2O
NH
4Cl+NaNO
2NaCl+N
2��+2H
2O
��
��2��ͼ�У�����������ͨ����װ�ã���װ�õ����ó��˽��������⣬����
�������壬�۲������ٶȣ����������͵���������
�������壬�۲������ٶȣ����������͵���������
��
��3�����ϳ�����������ȴ����������ͨ����װ�õ�ˮ�����հ�
����
����
�����ᡱ���ᡱ������������ԭ����
��������к��д���������ˮ�ĵ���������
��������к��д���������ˮ�ĵ���������
��
��4������װ������һ��ʱ�䰱����ͨ�������ͬʱ�������ȵIJ�˿������װ�õ���ƿ�ڣ���ʹ��˿���ֺ��ȵ�ԭ����
����������Ӧ��һ�����ȷ�Ӧ���ų�����ʹ��˿���ֺ���
����������Ӧ��һ�����ȷ�Ӧ���ų�����ʹ��˿���ֺ���
����ƿ�л��ɹ۲쵽�������ǣ�
�к���ɫ�������
�к���ɫ�������
��
��5��д����װ���а������Ļ�ѧ����ʽ��
��
��6����CH
4����ԭNO
2�������������������Ⱦ�����磮
CH
4��g��+4NO
2��g���T4NO��g��+CO
2��g��+2H
2O��g����H=-574kJ?mol
-1��
CH
4��g��+4NO��g���T2N
2��g��+CO
2��g��+2H
2O��g����H=-1160kJ?mol
-1��
�CH
4��ԭNO
2��N
2���Ȼ�ѧ����ʽ
CH4��g��+2N02��g��=N2��g��+C02��g��+2H20��g����H=-867KJ/mol
CH4��g��+2N02��g��=N2��g��+C02��g��+2H20��g����H=-867KJ/mol
�����ñ�״����4.48LCH
4��ԭNO
2��N
2����������ת�Ƶĵ�������Ϊ
1.6NA
1.6NA
�������ӵ�������ֵ��N
2��ʾ�����ų�������Ϊ
173.4
173.4
kJ��

 4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2 4NO+6H2O��
4NO+6H2O��