
”¾ĢāÄæ”潫2mol”¤L£1AĘųĢåŗĶ1mol”¤L£1BĘųĢå»ģŗĻ£¬²¢ŌŚŅ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗ2A(g)£«B(g)![]() 2C(g)£¬Čō¾2sŗó²āµĆCµÄÅضČĪŖ0.6mol”¤L£1£¬ĻÖÓŠĻĀĮŠ¼øÖÖĖµ·Ø£ŗ
2C(g)£¬Čō¾2sŗó²āµĆCµÄÅضČĪŖ0.6mol”¤L£1£¬ĻÖÓŠĻĀĮŠ¼øÖÖĖµ·Ø£ŗ
¢ŁÓĆĪļÖŹB±ķŹ¾µÄ·“Ó¦µÄĘ½¾łĖŁĀŹĪŖ0.6mol”¤L£1”¤s£1
¢ŚÓĆĪļÖŹA±ķŹ¾µÄ·“Ó¦µÄĘ½¾łĖŁĀŹĪŖ0.3mol”¤L£1”¤s£1
¢Ū2sŹ±ĪļÖŹAµÄ×Ŗ»ÆĀŹĪŖ70%
¢Ü2sŹ±ĪļÖŹBµÄÅضČĪŖ0.7mol”¤L£1
ĘäÖŠÕżČ·µÄŹĒ£Ø £©
A.¢Ł ¢ŪB.¢Ū ¢ÜC.¢Ś ¢ŪD.¢Ś ¢Ü

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪ¢ĮæŌŖĖŲÅšŗĶĆ¾¶ŌÖ²ĪļµÄŅ¶µÄÉś³¤ŗĶČĖĢå¹Ē÷ĄµÄ½”æµÓŠ×ÅŹ®·ÖÖŲŅŖµÄ×÷ÓĆ£¬Ęä»ÆŗĻĪļŅ²ÓŠ×Źć·ŗµÄÓ¦ÓĆ”£
(1)»łĢ¬BŌ×ӵļŪµē×ÓÅŲ¼Ķ¼ĪŖ_____________________£¬Ę䵌Ņ»µēĄėÄܱČBe__________£ØĢī”°“ó”±»ņ”°Š””±£©”£
(2)Čż¼ŪBŅ׊Ī³ÉÅäĄė×Ó£¬Čē[B(OH)4]£”¢[BH4]£µČ”£ÅäĄė×Ó[BH4]£µÄÖŠŠÄŌ×ÓµÄŌӻƷ½Ź½ĪŖ________£¬Š“³öÓėĘ仄ĪŖµČµē×ÓĢåµÄŅ»ÖÖŃōĄė×ÓŗĶŅ»ÖÖ·Ö×ӵĻÆѧŹ½£ŗ_________________”£
(3)ČżĀČ»ÆÅšµÄ·ŠµćĪŖ12.5”ę£¬¶ųĀČ»ÆĆ¾µÄ·ŠµćøßÓŚ1200”ę£¬ŌŅņŹĒ____________________________________”£
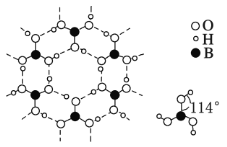
(4)ÅšĖį¾§ĢåŹĒʬד½į¹¹£¬ĻĀĶ¼±ķŹ¾µÄŹĒĘäÖŠŅ»²ćµÄ½į¹¹”£ĆæŅ»²ćÄŚ“ęŌŚµÄ×÷ÓĆĮ¦ÓŠ______________£»ÅšĖį¾§ĢåŌŚĄäĖ®ÖŠČܽā¶ČŗÜŠ”£¬µ«ŌŚČČĖ®ÖŠ½Ļ“ó£¬ŌŅņŹĒ _____________________________________________”£
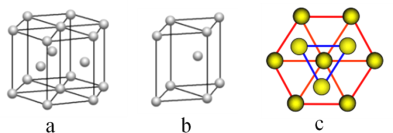
(5)Ć¾µ„ÖŹ¾§ĢåÖŠŌ×ӵĶѻżÄ£ŠĶČēĶ¼£¬ĖüµÄ¶Ń»żÄ£ŠĶĆū³ĘĪŖ__________________£»½ōĮŚµÄĖÄøöĆ¾Ō×ÓµÄÖŠŠÄĮ¬Ļß¹¹³ÉµÄÕżĖÄĆęĢå¼øŗĪĢåµÄĢå»żŹĒ2a cm3£¬Ć¾µ„ÖŹµÄĆܶČĪŖ¦Ń g”¤cm£3£¬ŅŃÖŖ°¢·üŁ¤µĀĀŽ³£ŹżĪŖNA£¬ŌņĆ¾µÄĦ¶ūÖŹĮæ¼ĘĖćŹ½ĪŖ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æµŖŃõ»ÆĪļŗĶĢ¼Ńõ»ÆŗĻĪļµÄ×ŪŗĻÖĪĄķŹĒµ±Ē°µÄÖŲŅŖæĪĢāÖ®Ņ»”£
ŅŃÖŖ£ŗ¢”.NO(g)+CO2(g)![]() NO2(g)+CO(g) ”÷H1=+234kJ”¤mol-1
NO2(g)+CO(g) ”÷H1=+234kJ”¤mol-1
¢¢.2NO(g)+2CO(g)![]() N2(g)+2CO2(g) ”÷H2=-745kJ”¤mol-1
N2(g)+2CO2(g) ”÷H2=-745kJ”¤mol-1
(1)NO2(g)ÓėCO(g)·“Ӧɜ³ÉĮ½ÖÖĪŽ¶¾ĘųĢåµÄČČ»Æѧ·½³ĢŹ½ĪŖ______________”£
(2)·“Ó¦¢”µÄÕż·“Ó¦µÄ»ī»ÆÄÜE_____”÷H1(Ģī”°>”±”¢”°<”±»ņ”°=”±)”£
(3)Ņ»¶ØŃ¹ĒæĻĀ£¬ĆܱÕČŻĘ÷ÖŠ·¢Éś·“Ó¦¢”ŗĶ·“Ó¦¢¢”£“ļµ½Ę½ŗāŗ󣬱£³ÖĘäĖūĢõ¼ž²»±ä£¬ÉżøßĪĀ¶Č£¬CO(g)µÄĢå»ż·ÖŹż________(Ģī”°Ōö“ó”±”¢”°¼õÉŁ”±»ņ”°ĪŽ·ØČ·¶Ø”±)£¬ŌŅņĪŖ_____________________”£
(4)ĻņĘšŹ¼ĪĀ¶ČĪŖt”ę”¢ČŻ»żĪŖ10LµÄŗćČŻ¾ųČȵÄĆܱÕČŻĘ÷ÖŠ³äČė2molNO(g)ŗĶ1molCO2(g)£¬·¢Éś·“Ó¦¢””£5minŹ±“ļµ½Ę½ŗā”£Ōņ£ŗ
¢ŁĻĀĮŠŹĀŹµÄÜĖµĆ÷øĆ·“Ó¦“ļµ½Ę½ŗāדĢ¬µÄŹĒ________(ĢīŃ”Ļī×ÖÄø)
A.»ģŗĻĘųĢåĪĀ¶Č²»±ä B.»ģŗĻĘųĢåµÄŃ¹Ēæ²»±ä
C. NO2ŗĶCOµÄÅضČÖ®±ČĪŖ1: 1 D.»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ²»±ä
¢Śt”ꏱ£¬ĻņĮķŅ»ČŻ»żĪŖ10 LµÄŗćĪĀŗćČŻµÄĆܱÕČŻĘ÷ÖŠ³äČė2 mol NO(g)ŗĶ1 mol CO2(g)£¬·¢Éś·“Ó¦i”£“ļµ½Ę½ŗāµÄŹ±¼ä______5 min(Ģī”°>”±”¢”°<”±»ņ”°=”±)”£
(5)ŌŚÄ³ĆܱÕČŻĘ÷ÖŠ³äÓŠ10 mol CO(g)ŗĶ20 mol NO(g)£¬·¢Éś·“Ó¦ii£¬COµÄĘ½ŗā×Ŗ»ÆĀŹ(¦Į)ÓėĪĀ¶Č(T)”¢Ń¹Ēæ(p)µÄ¹ŲĻµČēĶ¼ĖłŹ¾”£
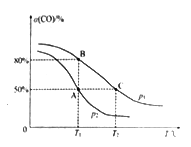
¢ŁA”¢B”¢C ČżµćµÄĘ½ŗā³£ŹżKA”¢KB”¢KCµÄ“󊔹ŲĻµĪŖ________£»p1ŗĶp2µÄ“󊔹ŲĻµĪŖ__________”£
¢ŚAµćŹ±.²āµĆČŻĘ÷µÄĢå»żĪŖ10 L£¬ŌņT1”ꏱ£¬øĆ·“Ó¦Ę½ŗā³£ŹżKµÄÖµĪŖ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
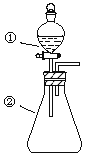
”¾ĢāÄæ”æĄūÓĆČēĶ¼×°ÖĆæÉŅŌŃéÖ¤·Ē½šŹōŠŌµÄ±ä»Æ¹ęĀÉ”£

(1)ŅĒĘ÷AµÄĆū³ĘĪŖ________£¬øÉŌļ¹ÜDµÄ×÷ÓĆŹĒ________________”£
(2)ŅŃÖŖŌŚ³£ĪĀĻĀ£¬øßĆĢĖį¼ŲŗĶÅØŃĪĖį·“Ó¦ÄÜÉś³ÉĀČĘų”£ŹµŃéŹŅÖŠĻÖÓŠŅ©Ę·Na2SČÜŅŗ”¢KMnO4”¢ÅØŃĪĖį”¢MnO2£¬ĒėŃ”ŌńŗĻŹŹŅ©Ę·Éč¼ĘŹµŃéŃéÖ¤ĀȵķĒ½šŹōŠŌ“óÓŚĮņµÄ£»×°ÖĆA”¢B”¢CÖŠĖł×°Ņ©Ę··Ö±šĪŖ________”¢________”¢________£¬×°ÖĆCÖŠµÄŹµŃéĻÖĻóĪŖ_________________£¬Ąė×Ó·½³ĢŹ½ĪŖ______”£
(3)ČōŅŖÖ¤Ć÷·Ē½šŹōŠŌ£ŗS>C>Si£¬ŌņAÖŠ¼Ó________”¢BÖŠ¼ÓNa2CO3”¢CÖŠ¼Ó________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¾ŪŗĻ¼īŹ½ĀČ»ÆĢś[![]() £ØmĪŖ¾ŪŗĻ¶Č£©ĪŖĪŽ»śøß·Ö×ÓŠõÄż¼Į£¬¹ć·ŗÓ¦ÓĆÓŚĖ®“¦Ąķ”£ŅŌĢśæóŹÆ”¢Ńõ»ÆĢśĘ¤»ņ
£ØmĪŖ¾ŪŗĻ¶Č£©ĪŖĪŽ»śøß·Ö×ÓŠõÄż¼Į£¬¹ć·ŗÓ¦ÓĆÓŚĖ®“¦Ąķ”£ŅŌĢśæóŹÆ”¢Ńõ»ÆĢśĘ¤»ņ![]() ĪŖŌĮĻ£¬ŌŚĖįŠŌĢõ¼žĻĀ¾Ńõ»Æ”¢Ė®½ā”¢¾ŪŗĻ”¢Źģ»ÆµČ²½Öč£¬æÉÖĘµĆ¾ŪŗĻ¼īŹ½ĀČ»ÆĢś”£
ĪŖŌĮĻ£¬ŌŚĖįŠŌĢõ¼žĻĀ¾Ńõ»Æ”¢Ė®½ā”¢¾ŪŗĻ”¢Źģ»ÆµČ²½Öč£¬æÉÖĘµĆ¾ŪŗĻ¼īŹ½ĀČ»ÆĢś”£
(1)øÖĢśĖįĻ“·ĻŅŗÖŠÖ÷ŅŖŗ¬ÓŠ![]() ŗĶ
ŗĶ![]() £¬ĶØČėæÕĘųæÉÖʵĆ
£¬ĶØČėæÕĘųæÉÖʵĆ![]() £¬·“Ó¦µÄ»Æѧ·½³ĢŹ½________”£Ńõ»Æ¼Į
£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½________”£Ńõ»Æ¼Į![]() Ņ²æÉŅŌ½«
Ņ²æÉŅŌ½«![]() Ńõ»Æ³É
Ńõ»Æ³É![]() £¬µ«“ęŌŚµÄȱµćŹĒ________________”£
£¬µ«“ęŌŚµÄȱµćŹĒ________________”£
(2)”°Ńõ»Æ”±¹ż³ĢÖŠ£¬ČōŃĪĖįµÄÅØ¶Č»ņĶ¶ČėĮæŌö“󣬳żĮĖ»į½µµĶ![]() µÄ×Ŗ»ÆĀŹ£¬»¹æÉÄܲśÉśµÄÓ°ĻģĪŖ________________”£
µÄ×Ŗ»ÆĀŹ£¬»¹æÉÄܲśÉśµÄÓ°ĻģĪŖ________________”£
(3)¢Ł×¼Č·³ĘČ”¾ŪŗĻ¼īŹ½ĀČ»ÆĢśŃłĘ·1.5000g£¬ÖĆÓŚ250mLµÄ׶ŠĪĘæÖŠ£¬¼ÓČėŹŹĮæĻ”ŃĪĖį£¬¼ÓČČ£¬ŃøĖŁ¼ÓČėÉŌ¹żĮæµÄ![]() ČÜŅŗ£Ø
ČÜŅŗ£Ø![]() ±»
±»![]() Ńõ»Æ³ÉĪŖ
Ńõ»Æ³ÉĪŖ![]() £©£¬³ä·Ö·“Ó¦ŗ󣬶ąÓąµÄ
£©£¬³ä·Ö·“Ó¦ŗ󣬶ąÓąµÄ![]() ÓĆ
ÓĆ![]() Ńõ»Æ³żČ„”£ŌŁ¼ÓČėŹŹĮæµÄ
Ńõ»Æ³żČ„”£ŌŁ¼ÓČėŹŹĮæµÄ![]() ×é³ÉµÄ»ģĖį¼°4~5µĪÖøŹ¾¼Į£¬ÓĆ
×é³ÉµÄ»ģĖį¼°4~5µĪÖøŹ¾¼Į£¬ÓĆ![]() ČÜŅŗµĪ¶ØÖĮÖÕµć£ØµĪ¶Ø¹ż³ĢÖŠ
ČÜŅŗµĪ¶ØÖĮÖÕµć£ØµĪ¶Ø¹ż³ĢÖŠ![]() Óė
Óė![]() ·“Ӧɜ³É
·“Ӧɜ³É![]() ŗĶ
ŗĶ![]() £©£¬ĻūŗÄ
£©£¬ĻūŗÄ![]() ČÜŅŗ
ČÜŅŗ![]() ”£
ӣ
¢ŚĮķ³ĘČ”µČÖŹĮæµÄ¾ŪŗĻ¼īŹ½ĀČ»ÆĢśŃłĘ·ČÜÓŚĖ®ÖŠ£¬Åä³É500mLČÜŅŗ£¬Č”25mLČÜŅŗӌ׶ŠĪĘæÖŠ£¬ÓĆøõĖį¼Ų£Ø![]() £©ČÜŅŗ×÷ÖøŹ¾¼Į£¬ÓĆ
£©ČÜŅŗ×÷ÖøŹ¾¼Į£¬ÓĆ![]() ±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄ
±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄ![]() ČÜŅŗ8.10mL”£
ČÜŅŗ8.10mL”£
¼ĘĖćøĆѳʷ֊ĀČŌŖĖŲŗĶĢśŌŖĖŲµÄÖŹĮæ·ÖŹżÖ®±Č![]() ĪŖ________£ØŠ“³ö¼ĘĖć¹ż³Ģ£¬½į¹ū±£ĮōĖÄĪ»ÓŠŠ§Źż×Ö£©”£
ĪŖ________£ØŠ“³ö¼ĘĖć¹ż³Ģ£¬½į¹ū±£ĮōĖÄĪ»ÓŠŠ§Źż×Ö£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©Š“³öĻĀĮŠĪļÖŹ×Ŗ»ÆµÄ»Æѧ·½³ĢŹ½”£

¢Ł_____________________________________________£¬
¢Ś_____________________________________________£¬
¢Ū_____________________________________________£¬
£Ø2£©Š“³öĻĀĮŠĪļÖŹ×Ŗ»ÆµÄ»Æѧ·½³ĢŹ½”£

¢Ł_____________________________________________£¬
¢Ś_____________________________________________£¬
¢Ū_____________________________________________£¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÉčNAĪŖ°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø £©
A.32g S8ÖŠSŹżÄæĪŖNA
B.71gĀČĘųĶØČė×ćĮæµÄNaOHČÜŅŗÖŠ×ŖŅʵē×ÓŹżÄæĪŖNA
C.100mLÅضČĪŖ0.1mol/LµÄĆ÷·ÆČÜŅŗÖŠ£¬SO42-ŹżÄæĪŖ0.02NA
D.11.2L Cl2ČÜÓŚĖ®£¬ČÜŅŗÖŠCl-”¢ClO-ŗĶHClOµÄĪ¢Į£ŹżÖ®ŗĶŅ»¶ØĪŖNA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖ£ŗN2(g)£«3H2(g) ![]() 2 NH3(g) ¦¤H£½£92.4 kJ”¤mol£1”£Ņ»¶ØĢõ¼žĻĀ£¬ĻÖÓŠČŻ»żĻąĶ¬ĒŅŗćČŻµÄĆܱÕČŻĘ÷¼×ÓėŅŅ£ŗ¢Ł Ļņ¼×ÖŠĶØČė1 mol N2ŗĶ3 mol H2£¬“ļµ½Ę½ŗāŹ±·Å³öČČĮæQ1 kJ£»¢Ś ĻņŅŅÖŠĶØČė0.5 mol N2ŗĶ1.5 mol H2£¬“ļµ½Ę½ŗāŹ±·Å³öČČĮæQ2 kJ”£ŌņĻĀĮŠ¹ŲĻµŹ½ÕżČ·µÄŹĒ
2 NH3(g) ¦¤H£½£92.4 kJ”¤mol£1”£Ņ»¶ØĢõ¼žĻĀ£¬ĻÖÓŠČŻ»żĻąĶ¬ĒŅŗćČŻµÄĆܱÕČŻĘ÷¼×ÓėŅŅ£ŗ¢Ł Ļņ¼×ÖŠĶØČė1 mol N2ŗĶ3 mol H2£¬“ļµ½Ę½ŗāŹ±·Å³öČČĮæQ1 kJ£»¢Ś ĻņŅŅÖŠĶØČė0.5 mol N2ŗĶ1.5 mol H2£¬“ļµ½Ę½ŗāŹ±·Å³öČČĮæQ2 kJ”£ŌņĻĀĮŠ¹ŲĻµŹ½ÕżČ·µÄŹĒ
A. Q1£½2Q2£½92.4B. 92.4£½Q1£¼2Q2
C. 92.4£¾Ql£¾2Q2D. Q1£½2Q2£¼92.4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠĻĀĮŠŹµŃé£ŗ½«¢ŁÖŠČÜŅŗµĪČė¢ŚÖŠ£¬Ō¤²āµÄĻÖĻóÓėŹµ¼ŹĻą·ūµÄŹĒ
Ń”Ļī | ¢ŁÖŠĪļÖŹ | ¢ŚÖŠĪļÖŹ | Ō¤²ā¢ŚÖŠµÄĻÖĻó |
A | Ļ”ŃĪĖį | Ģ¼ĖįÄĘÓėĒāŃõ»ÆÄʵĻģŗĻČÜŅŗ | Į¢¼“²śÉśĘųÅŻ |
B | ÅØĻõĖį | ÓĆÉ°Ö½“ņÄ„¹żµÄĀĮĢõ | ²śÉśŗģ×ŲÉ«ĘųĢå |
C | ŠĀÖĘĀČĖ® | µķ·Ūµā»Æ¼ŲČÜŅŗ | ČÜŅŗ±äĄ¶É« |
D | ÅØŃĪĖį | MnO2 | ²śÉś»ĘĀĢÉ«ĘųĢå |
A.AB.BC.CD.D
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com