
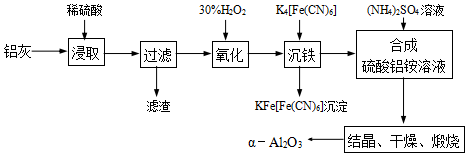

���� ���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3����ϡ���ᣬAl2O3��FeO��Fe2O3ת��Ϊ���ӣ�SiO2���������ᣬ���ˣ���Һ�к���Al3+��Fe2+��Fe3+����˫��ˮ��Fe2+������ΪFe3+������K4[Fe��CN��6]Fe3+ת��Ϊ���������ˣ�����Һ�м�������泥�����NH4Al��SO4��2���ᾧ��������յõ���-Al2O3��
��1��Al2O3�����ᷴӦ������������ˮ��
��2����ʵ�������У�H2O2���������ԣ������������������������ӣ��¶Ȳ��ܹ��߷�������������ȷֽ⣻
��3����NH4Al��SO4��2•12H2O�ֽ����ɵ�����NH3��SO3�������������գ�����������������գ�
��NH3��SO3�ܱ�����������Һ���գ�
��KMnO4���������Ӧ������������Ӻ������ӣ�
��� �⣺���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3����ϡ���ᣬAl2O3��FeO��Fe2O3ת��Ϊ���ӣ�SiO2���������ᣬ���ˣ���Һ�к���Al3+��Fe2+��Fe3+����˫��ˮ��Fe2+������ΪFe3+������K4[Fe��CN��6]Fe3+ת��Ϊ���������ˣ�����Һ�м�������泥�����NH4Al��SO4��2���ᾧ��������յõ���-Al2O3��
��1��Al2O3�����ᷴӦ������������ˮ���䷴Ӧ�ķ���ʽΪ��Al2O3+3H2SO4=Al2��SO4��3+3H2O��
�ʴ�Ϊ��Al2O3+3H2SO4=Al2��SO4��3+3H2O��
��2����Һ�к���Al3+��Fe2+��Fe3+����30%��H2O2��ҺFe2+������ΪFe3+���䷴Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O���÷�Ӧ������¶ȵ���40�棬��Ŀ���Ƿ�ֹH2O2�ֽ⣬
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O����ֹH2O2�ֽ⣻
��3����NH4Al��SO4��2•12H2O�ֽ����ɵ�����NH3��SO3�������������գ�����������������գ����������ƿ���ռ�����������N2��
�ʴ�Ϊ��N2��
��NH3��������ˮ��NH3�����ܱ�����������Һ���գ�SO3��ˮ��Ӧ�����ᣬ��SO3Ҳ������������Һ���գ�������������NaHSO3��Һ���յ����ʳ���H2O��g�����SO3��NH3��
�ʴ�Ϊ��SO3��NH3��
�����������£�KMnO4���������Ӧ������������Ӻ������ӣ��䷴Ӧ�����ӷ���ʽΪ��2MnO4-+5SO2+2H2O=2Mn2++5SO42-+4H+��
�ʴ�Ϊ��2MnO4-+5SO2+2H2O=2Mn2++5SO42-+4H+��
���� ��������������ȡ��������Ϊ���壬����Ԫ�ػ��������ʼ��ת����������ԭ�����ӷ���ʽ��ʵ�����������֪ʶ�㣬ע������Ʊ�ԭ�����������ʵ������Լ���ط�Ӧ����ʽ����д����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����������
����������� ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������ϡ���FeO+2H+�TFe2++H2O | |
| B�� | ��CaCl2��Һ��ͨ��������CO2��Ca2++CO2+H2O=CaCO3��+2H+ | |
| C�� | ̼�������Һ�м����������������Һ��HCO3-+OH-=CO32-+H2O | |
| D�� | �����ʯ��ˮ�еμ�������NaHCO3��Һ��Ca2++OH-+HCO3-=CaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
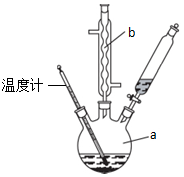 ������ͭ�Ǻϳ��������������в���--��������ͭ����Ҫǰ����֮һ��
������ͭ�Ǻϳ��������������в���--��������ͭ����Ҫǰ����֮һ��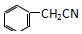 +2H2O+H2SO4$\stackrel{100-130��}{��}$+NH4HSO4
+2H2O+H2SO4$\stackrel{100-130��}{��}$+NH4HSO4 ������ܼ����Ҵ�������������������ܽ�ȣ����ڳ�ַ�Ӧ��
������ܼ����Ҵ�������������������ܽ�ȣ����ڳ�ַ�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
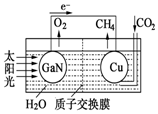 ��ѧ���õ����ز�����ͭ��װ��ͼ���˹����ϵͳ�����ø�װ�óɹ���ʵ������CO2��H2O�ϳ�CH4��
��ѧ���õ����ز�����ͭ��װ��ͼ���˹����ϵͳ�����ø�װ�óɹ���ʵ������CO2��H2O�ϳ�CH4���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com