
 ĆææĪ±ŲĮ·ĻµĮŠ“š°ø
ĆææĪ±ŲĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| “¼ |
| ”÷ |
| “¼ |
| ”÷ |
| Ė® |
| ”÷ |
| Ė® |
| ”÷ |


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
”¶ĪļÖŹ½į¹¹ÓėŠŌÖŹ”·Ä£æéŃ”ŌńĢā
1.ĻĀĮŠŠŌÖŹ²»ÄÜÓĆ½šŹō¼üĄķĀŪ½āŹĶµÄŹĒ
A.µ¼µēŠŌ B.µ¼ČČŠŌ C.ŃÓÕ¹ŠŌ D.ŠāŹ“ŠŌ
2.ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ
A.O3ÓėSO2µÄ½į¹¹ÓėŠŌÖŹĻąĖĘ
B.Įņ·Ū²»ČÜÓŚĖ®£¬Ņ×ČÜÓŚCS2ÖŠ
C.Be£ØOH£©2ŹĒĮ½ŠŌĒāŃõ»ÆĪļ
D.ŌŚĻąĶ¬Ģõ¼žĻĀ£¬ µÄ·ŠµćøßÓŚ
µÄ·ŠµćøßÓŚ![]()
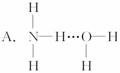
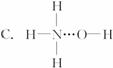
3.°±ĘųČÜÓŚĖ®Ź±£¬“ó²æ·ÖNH3ÓėH2OŅŌĒā¼ü£ØÓĆ”°””±±ķŹ¾£©½įŗĻŠĪ³ÉNH3”¤H2O·Ö×Ó£¬øł¾Ż°±Ė®µÄŠŌÖŹæÉĶĘÖŖNH3”¤H2OµÄ½į¹¹Ź½ĪŖ


”¶ÓŠ»ś»Æѧ»ł“””·Ä£æéŃ”ŌńĢā
4.ŅŃÖŖijӊ»śĪļAµÄ·Ö×ÓŹ½C2H6OµÄŗģĶā¹āĘ×ŗĶŗĖ“Ź²ÕńĒāĘ×ČēĻĀĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·ØÖŠ“ķĪóµÄŹĒ
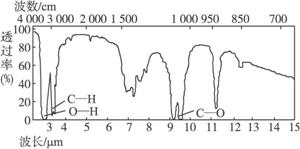
Ī“ÖŖĪļAµÄŗģĶā¹āĘ×
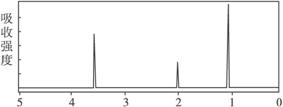
Ī“ÖŖĪļAµÄŗĖ“Ź²ÕńĒāĘ×
A.ÓÉŗģĶā¹āĘ×æÉÖŖ£¬øĆÓŠ»śĪļÖŠÖĮÉŁŗ¬ÓŠČżÖÖ²»Ķ¬µÄ»Æѧ¼ü
B.ÓÉŗĖ“Ź²ÕńĒāĘ×æÉÖŖ£¬øĆÓŠ»śĪļ·Ö×ÓÖŠÓŠČżÖÖ²»Ķ¬µÄĒāŌ×ÓĒŅøöŹż±ČĪŖ1”Ć2”Ć3
C.½öÓÉĘäŗĖ“Ź²ÕńĒāĘ×æÉÖŖĘä·Ö×ÓÖŠµÄø÷ĄąŠĶĒāŌ×Ó×ÜŹż
D.ŌņAµÄ½į¹¹¼ņŹ½ĪŖCH3”ŖO”ŖCH3
5.ŌŚ°¢Ė¾Ę„ĮֵĽį¹¹¼ņŹ½£ØĻĀŹ½£©ÖŠ¢Ł¢Ś¢Ū¢Ü¢Ż¢Ž·Ö±š±ź³öĮĖĘä·Ö×ÓÖŠµÄ²»Ķ¬µÄ¼ü”£½«°¢Ė¾Ę„ĮÖÓė×ćĮæNaOHČÜŅŗ¹²ÖóŹ±£¬·¢Éś·“Ó¦Ź±¶Ļ¼üµÄĪ»ÖĆŹĒ
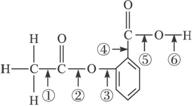
A.¢Ł¢Ü B.¢Ś¢Ż C.¢Ū¢Ü D.¢Ś¢Ž
6.¾Ł°ģ”°ČĖĪÄ°ĀŌĖ”±µÄŅ»øöÖŲŅŖĢåĻÖ¾ĶŹĒ½ūÖ¹ŌĖ¶ÆŌ±·žÓĆŠĖ·Ü¼Į”£ÓŠŅ»ÖÖŠĖ·Ü¼ĮµÄ½į¹¹¼ņŹ½ČēĻĀŹ½£¬ĻĀĮŠÓŠ¹ŲøĆĪļÖŹµÄĖµ·ØÕżČ·µÄŹĒ
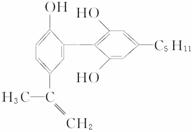
A.øĆ·Ö×ÓÖŠĖłÓŠĢ¼Ō×ÓæÉŅŌĪČ¶ØµŲ¹²“ęŌŚŅ»øöĘ½ĆęÖŠ
B.1 moløĆĪļÖŹÓėÅØäåĖ®ŗĶH2·“Ó¦Ź±£¬×ī¶ąĻūŗÄBr2ŗĶH2µÄĪļÖŹµÄĮæ·Ö±šĪŖ4 mol”¢7 mol
C.ÓöFeCl3ČÜŅŗĻŌ×ĻÉ«£¬ŅņĪŖøĆĪļÖŹÓė±½·ÓŹōÓŚĶ¬ĻµĪļ
D.µĪČėĖįŠŌKMnO4ČÜŅŗ£¬¹Ū²ģµ½×ĻÉ«ĶŹČ„£¬æÉÖ¤Ć÷·Ö×ÓÖŠ“ęŌŚĖ«¼ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÖŲĒģŹŠŃī¼ŅĘŗ֊ѧøßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
ŅŃÖŖijĘųĢ¬»ÆŹÆČ¼ĮĻXÖŠÖ»ŗ¬ÓŠĢ¼”¢ĒāĮ½ÖÖŌŖĖŲ£¬ĪŖĢ½¾æøĆĘųĢåÖŠĢ¼ŗĶĒāĮ½ÖÖŌŖĖŲµÄÖŹĮæ±Č£¬Ä³Ķ¬Ń§Éč¼ĘĮĖČ¼ÉշزāĮæµÄŹµŃé·½°ø£¬Ķعż²āĮæ×°ÖĆCŗĶDµÄŌöÖŲ¼“æÉĒóµĆĢ¼ŗĶĒāĮ½ÖÖŌŖĖŲµÄÖŹĮæ±Č”£ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾(ŅŃÖŖCuOæÉŅŌ×÷ĪŖĢ¼Ēā»ÆŗĻĪļČ¼Éյē߻ƼĮ)£ŗ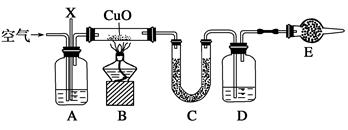
(1)ČōA×°ÖĆÖŠĖłŹ¢·ÅµÄŅ©Ę·ŹĒÅØNaOH ČÜŅŗ£¬Š“³ö×°ÖĆAµÄŅ»ÖÖ×÷ÓĆ£ŗ_
(2)C×°ÖĆÖŠĖłŹ¢·ÅµÄŅ©Ę·ŹĒ£ŗ
(3)D×°ÖĆÖŠĖłŹ¢·ÅµÄŅ©Ę·ŹĒ£ŗ
(4)E×°ÖĆÖŠĖłŹ¢·ÅµÄŅ©Ę·ŹĒ£ŗ
(5)ÉĻŹö×°ÖĆÖŠÓŠŅ»“¦“ķĪó£¬ (²»æ¼ĀĒ
¾Ę¾«µĘŗĶ¼ÓČČ·½·ØæÉÄÜ“ęŌŚµÄ“ķĪó£»
ČōŌö¼ÓŅĒĘ÷ŗĶŅ©Ę·£¬ĒėÖøĆ÷ŅĒĘ÷”¢Ņ©
Ę·Ćū³ĘŗĶĪ»ÖĆ)£ŗĘäøÄÕż·½·ØŹĒ
(6) ČōŹµŃé×°ÖĆ¾¹żøÄÕżŗ󣬽ųŠŠČēĻĀ¶ØĮæŹµŃé£ŗ×¼Č·³ĘČ”7.2 gѳʷ£ØÖ»ŗ¬C”¢H”¢OČżÖÖŌŖĖŲÖŠµÄĮ½ÖÖ»ņČżÖÖ£©£¬¾³ä·ÖČ¼ÉÕŗó£¬UŠĪ¹ÜC¹ÜÖŹĮæŌö¼Ó10.8 g£¬¹ćæŚĘæDÖŹĮæŌö¼Ó22 g£¬ŌņøĆÓŠ»śĪļµÄ×ī¼ņŹ½ĪŖ
£Ø7£©ČōÄÜČ·¶ØĘä·Ö×ÓŹ½£¬ŌņĘäĶ¬·ÖŅģ¹¹ĢåÖŠ·Šµć×īµĶĪļÖŹµÄĆū³Ę__ ”” (Ļ°¹ßĆüĆū·Ø)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģÖŲĒģŹŠøßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
ŅŃÖŖijĘųĢ¬»ÆŹÆČ¼ĮĻXÖŠÖ»ŗ¬ÓŠĢ¼”¢ĒāĮ½ÖÖŌŖĖŲ£¬ĪŖĢ½¾æøĆĘųĢåÖŠĢ¼ŗĶĒāĮ½ÖÖŌŖĖŲµÄÖŹĮæ±Č£¬Ä³Ķ¬Ń§Éč¼ĘĮĖČ¼ÉշزāĮæµÄŹµŃé·½°ø£¬Ķعż²āĮæ×°ÖĆCŗĶDµÄŌöÖŲ¼“æÉĒóµĆĢ¼ŗĶĒāĮ½ÖÖŌŖĖŲµÄÖŹĮæ±Č”£ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾(ŅŃÖŖCuOæÉŅŌ×÷ĪŖĢ¼Ēā»ÆŗĻĪļČ¼Éյē߻ƼĮ)£ŗ

(1)ČōA×°ÖĆÖŠĖłŹ¢·ÅµÄŅ©Ę·ŹĒÅØNaOH ČÜŅŗ£¬Š“³ö×°ÖĆAµÄŅ»ÖÖ×÷ÓĆ£ŗ_
(2)C×°ÖĆÖŠĖłŹ¢·ÅµÄŅ©Ę·ŹĒ£ŗ
(3)D×°ÖĆÖŠĖłŹ¢·ÅµÄŅ©Ę·ŹĒ£ŗ
(4)E×°ÖĆÖŠĖłŹ¢·ÅµÄŅ©Ę·ŹĒ£ŗ
(5)ÉĻŹö×°ÖĆÖŠÓŠŅ»“¦“ķĪó£¬ (²»æ¼ĀĒ
¾Ę¾«µĘŗĶ¼ÓČČ·½·ØæÉÄÜ“ęŌŚµÄ“ķĪó£»
ČōŌö¼ÓŅĒĘ÷ŗĶŅ©Ę·£¬ĒėÖøĆ÷ŅĒĘ÷”¢Ņ©
Ę·Ćū³ĘŗĶĪ»ÖĆ)£ŗĘäøÄÕż·½·ØŹĒ
(6) ČōŹµŃé×°ÖĆ¾¹żøÄÕżŗ󣬽ųŠŠČēĻĀ¶ØĮæŹµŃé£ŗ ×¼Č·³ĘČ”7.2 gѳʷ£ØÖ»ŗ¬C”¢H”¢OČżÖÖŌŖĖŲÖŠµÄĮ½ÖÖ»ņČżÖÖ£©£¬¾³ä·ÖČ¼ÉÕŗó£¬UŠĪ¹ÜC¹ÜÖŹĮæŌö¼Ó10.8 g£¬¹ćæŚĘæDÖŹĮæŌö¼Ó22 g£¬ŌņøĆÓŠ»śĪļµÄ×ī¼ņŹ½ĪŖ
£Ø7£©ČōÄÜČ·¶ØĘä·Ö×ÓŹ½£¬ŌņĘäĶ¬·ÖŅģ¹¹ĢåÖŠ·Šµć×īµĶĪļÖŹµÄĆū³Ę__ ”” (Ļ°¹ßĆüĆū·Ø)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
£Ø6·Ö£©ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ £ØĢīŠņŗÅ£©”£
¢ŁÓÉÓŚµāŌŚ¾Ę¾«ÖŠµÄČܽā¶Č“ó£¬ĖłŅŌæÉÓĆ¾Ę¾«½«µāĖ®ÖŠµÄµāŻĶČ”³öĄ“
¢ŚĖ®µÄ·ŠµćŹĒ100”ę£¬¾Ę¾«µÄ·ŠµćŹĒ78.5”ę£¬ÓĆÖ±½ÓÕōĮó·ØÄÜŹ¹ŗ¬Ė®¾Ę¾«±äĪŖĪŽĖ®¾Ę¾«
¢ŪÓÉÓŚ½ŗĮ£µÄÖ±¾¶±ČĄė×Ó“ó£¬ĖłŅŌµķ·ŪČÜŅŗÖŠ»ģÓŠµÄµā»Æ¼ŲæÉÓĆÉųĪö·Ø·ÖĄė
¢Ü·ÖĄė±½ŗĶ±½·ÓµÄ»ģŗĻŅŗ£¬ĻČ¼ÓČėŹŹĮæÅØäåĖ®£¬ŌŁ¹żĀĖ”¢·ÖŅŗ£¬¼“æÉŹµĻÖ
¢ŻÓÉÓŚøß¼¶Ö¬·¾ĖįÄĘŃĪŌŚĖ®ÖŠµÄ·ÖÉ¢ÖŹĪ¢Į£Ö±¾¶ŌŚ1nm”«100 nmÖ®¼ä£¬ĖłŅŌæÉÓĆŹ³ŃĪŹ¹øß¼¶Ö¬·¾ĖįÄÉ“ÓŌķ»Æ·“Ó¦ŗóµÄ»ģŗĻĪļÖŠĪö³ö
¢Ž²»É÷°Ń±½·ÓČÜŅŗÕ“µ½Ę¤·ōÉĻ£¬Ó¦Į¢¼“ÓĆ¾Ę¾«Ļ“µÓ
¢ßÓĆĻ”äåĖ®µĪČė±½·ÓČÜŅŗÖŠÖʱø2£¬4£¬6£Čżäå±½·Ó
¢ąŹµŃéŹŅŹ¹ÓĆĢå»ż±ČĪŖ1:3µÄÅØĮņĖįÓėŅŅ“¼µÄ»ģŗĻČÜŅŗÖĘŅŅĻ©Ź±£¬ĪŖ·Ą¼ÓČČŹ±·“Ó¦»ģŗĻŅŗ³öĻÖ±©·ŠĻÖĻ󣬳żĮĖŅŖ¼Ó·ŠŹÆĶā£¬»¹Ó¦×¢Ņā»ŗĀż¼ÓČČČĆĪĀ¶ČĀżĀżÉżÖĮ170”ę
17£®.(18·Ö)ij»ÆѧŠ”×é²ÉÓĆĄąĖĘÖĘŅŅĖįŅŅõ„µÄ×°ÖĆ£ØČēÓŅĶ¼£©£¬ŅŌ»·¼ŗ“¼Öʱø»·¼ŗĻ©£ŗ
 ŅŃÖŖ£ŗ
ŅŃÖŖ£ŗ
| ĆÜ¶Č | ČŪµć | ·Šµć | ČܽāŠŌ | |
| »·¼ŗ“¼ | 0.96 | 25 | 161 | ÄÜČÜÓŚĖ® |
| »·¼ŗĻ© | 0.81 | £103 | 83 | ÄŃČÜÓŚĖ® |
£Ø1£©Öʱø“ÖĘ·
½«12.5mL»·¼ŗ“¼¼ÓČėŹŌ¹ÜAÖŠ£¬ŌŁ¼ÓČė1mLÅØĮņĖį£¬Ņ”ŌČŗó·ÅČėĖé“Éʬ£¬»ŗĀż¼ÓČČÖĮ·“Ó¦ĶźČ«£¬ŌŚŹŌ¹ÜCÄŚµĆµ½»·¼ŗĻ©“ÖĘ·”£
¢ŁAÖŠĖé“ÉʬµÄ×÷ÓĆŹĒ £¬
µ¼¹ÜB³żĮĖµ¼ĘųĶā»¹¾ßÓŠµÄ×÷ÓĆŹĒ ”£
¢ŚŹŌ¹ÜCÖĆÓŚ±łĖ®Ō”ÖŠµÄÄæµÄŹĒ ”£
 £Ø2£©Öʱø¾«Ę·
£Ø2£©Öʱø¾«Ę·
¢Ł»·¼ŗĻ©“ÖĘ·ÖŠŗ¬ÓŠ»·¼ŗ“¼ŗĶÉŁĮæĖįŠŌŌÓÖŹµČ”£¼ÓČė±„ŗĶ
Ź³ŃĪĖ®£¬Õńµ“”¢¾²ÖĆ”¢·Ö²ć£¬»·¼ŗĻ©ŌŚ ²ć£ØĢī
”°ÉĻ”±»ņ”°ĻĀ”±£©£¬·ÖŅŗŗóÓĆ £ØĢīČė±ąŗÅ£©Ļ“µÓ”£
A£®KMnO4ČÜŅŗ B£®Ļ”H2SO4 C£®Na2CO3ČÜŅŗ
¢ŚŌŁ½«»·¼ŗĻ©°“ÓŅĶ¼×°ÖĆÕōĮó£¬ĄäČ“Ė®“Ó æŚ½ųČė”£
ÕōĮóŹ±ŅŖ¼ÓČėÉśŹÆ»Ņ£¬ÄæµÄŹĒ:”””” ”””” ”£
¢ŪŹÕ¼Æ²śĘ·Ź±£¬æŲÖʵÄĪĀ¶ČÓ¦ŌŚ ×óÓŅ£¬ŹµŃéÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæµĶÓŚĄķĀŪ²śĮ棬æÉÄܵÄŌŅņŹĒ £Ø £©
A£®ÕōĮ󏱓Ó70”ęæŖŹ¼ŹÕ¼Æ²śĘ· B£®»·¼ŗ“¼Źµ¼ŹÓĆĮæ¶ąĮĖ
C£®Öʱø“ÖĘ·Ź±»·¼ŗ“¼Ėę²śĘ·Ņ»ĘšÕō³ö
£Ø3£©ŅŌĻĀĒų·Ö»·¼ŗĻ©¾«Ę·ŗĶ“ÖĘ·µÄ·½·Ø£¬ŗĻĄķµÄŹĒ ( )
A£®ÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ B£®ÓĆ½šŹōÄĘ C£®²ā¶Ø·Šµć
18£®£Ø12·Ö£©ÓŠ»ś
ĪļAµÄ½į¹¹¼ņŹ½ĪŖ![]() £¬ĖüæÉĶعż²»Ķ¬»Æѧ·“Ó¦·Ö±šÖʵĆB”¢C”¢DŗĶEĖÄÖÖĪļÖŹ”£
£¬ĖüæÉĶعż²»Ķ¬»Æѧ·“Ó¦·Ö±šÖʵĆB”¢C”¢DŗĶEĖÄÖÖĪļÖŹ”£

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Öø³ö·“Ó¦µÄĄąŠĶ£ŗA”śC£ŗ ”£
£Ø2£©ŌŚA~EĪåÖÖĪļÖŹÖŠ£¬»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄŹĒ £ØĢī“śŗÅ£©”£
£Ø3£©Š“³öÓÉAÉś³ÉBµÄ»Æѧ·½³ĢŹ½
ӣ
£Ø4£©ŅŃÖŖHCHO·Ö×ÓÖŠĖłÓŠŌ×Ó¶¼ŌŚĶ¬Ņ»Ę½ĆęÄŚ£¬ŌņŌŚÉĻŹö·Ö×ÓÖŠĖłÓŠµÄŌ×ÓÓŠæÉÄܶ¼ŌŚĶ¬Ņ»Ę½ĆęµÄĪļÖŹŹĒ £ØĢīŠņŗÅ£©”£
£Ø5£©CÄÜŠĪ³Éøß¾ŪĪļ£¬øĆøß¾ŪĪļµÄ½į¹¹¼ņŹ½ĪŖ ”£
£Ø6£©Š“³öDÓėNaOHČÜŅŗ¹²ČČ·“Ó¦µÄ»Æѧ·½³ĢŹ½
ӣ
19.£Ø16·Ö£©Čā
¹šĖį¼×õ„£Ø £©³£ÓĆÓŚµ÷ÖĘ¾ßÓŠ²ŻŻ®”¢ĘĻĢŃ”¢Ó£ĢŅ”¢Ļć×ÓĄ¼µČĻćĪ¶µÄŹ³ÓĆĻć¾«
£©³£ÓĆÓŚµ÷ÖĘ¾ßÓŠ²ŻŻ®”¢ĘĻĢŃ”¢Ó£ĢŅ”¢Ļć×ÓĄ¼µČĻćĪ¶µÄŹ³ÓĆĻć¾«
¢ÅČā¹šĖį¼×õ„µÄ·Ö×ÓŹ½ŹĒ £»
 ¢ĘĻĀĮŠÓŠ¹ŲČā¹šĖį¼×õ„µÄŠšŹöÖŠ£¬ÕżČ·µÄŹĒ Ģī×ÖÄø£©£»
¢ĘĻĀĮŠÓŠ¹ŲČā¹šĖį¼×õ„µÄŠšŹöÖŠ£¬ÕżČ·µÄŹĒ Ģī×ÖÄø£©£»
A£®ÄÜÓėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·¢Éś¼Ó³É·“Ó¦
B£®ĪŽ·ØŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«
C£®ŌŚ¼īŠŌĢõ¼žĻĀÄÜ·¢ÉśĖ®½ā·“Ó¦
D£®²»æÉÄÜ·¢Éś¼Ó¾Ū·“Ó¦
¢ĒGĪŖČā¹šĖį¼×õ„µÄŅ»ÖÖĶ¬·ÖŅģ¹¹Ģ壬Ęä·Ö×Ó½į¹¹Ä£ŠĶČēÓŅĶ¼ĖłŹ¾£ØĶ¼ÖŠĒņÓėĒņÖ®¼äĮ¬Ļß±ķŹ¾µ„¼ü»ņĖ«¼ü£©”£ŌņGµÄ½į¹¹¼ņŹ½ĪŖ £»
¢ČÓĆ·¼ĻćĢžAĪŖŌĮĻŗĻ³ÉGµÄĀ·ĻßČēĻĀ£ŗ

¢Ł»ÆŗĻĪļEÖŠµÄ¹ŁÄÜĶÅÓŠ £ØĢīĆū³Ę£©”£
¢ŚF”śGµÄ·“Ó¦ĄąŠĶŹĒ £¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ _ _ ”£
¢ŪC”śDµÄ»Æѧ·½³ĢŹ½ĪŖ _”£
¢ÜŠ“³ö·ūŗĻĻĀĮŠĢõ¼žµÄFµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ ”£O%M
¢”£®·Ö×ÓÄŚŗ¬±½»·£¬ĒŅ±½»·ÉĻÖ»ÓŠŅ»øöÖ§Į“£»
¢¢£®Ņ»¶ØĢõ¼žĻĀ£¬1moløĆĪļÖŹÓė×ćĮæŅų°±ČÜŅŗ³ä·Ö·“Ó¦£¬Éś³É4molŅųµ„ÖŹ”£
20£®£Ø10·Ö£©ÓŠ»śĪļAµÄÕōĘū¶ŌĶ¬ĪĀĶ¬Ń¹ĻĀĒāĘųµÄĻą¶ŌĆܶČĪŖ31£¬Č”3.1æĖAĪļÖŹŌŚ×ćĮæŃõĘųÖŠ³ä·ÖČ¼ÉÕ£¬Ö»Éś³É2.7æĖĖ®ŗĶ±ź×¼×“æöĻĀCO22.24L£¬ĒóÓŠ»śĪļµÄ·Ö×ÓŹ½£»ČōøĆÓŠ»śĪļ0.2molĒ”ŗĆÓė9.2æĖ½šŹōÄĘĶźČ«·“Ó¦£¬ĒėŠ“³öÓŠ»śĪļµÄ½į¹¹¼ņŹ½²¢ĆüĆū”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com