
�����ʽṹ�����ʡ�
X��Y��Z��M��N��QΪԪ�����ڱ�ǰ�����ڵ�����Ԫ�ء�����Xԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�Yԭ�Ӻ����L���������K���������Z�ǵؿ��ں�����������������ߵ�Ԫ�أ�M���ڲ��������������������9����N��ԭ��������MС1, Q��Ԫ�����ڱ��ĸ�Ԫ���е縺�������ش��������⣺
��1��XԪ�������ڱ��е�λ���� ���� ��Ԫ�أ������������ӵĵ����Ų�ͼΪ ��
��2��XZ2���ӵ�����ṹ�� ��YZ2������Y���ӻ��������Ϊ ����ͬ������������ˮ�е��ܽ�Ƚϴ���� ��д����ʽ���������� ��
��3������Ԫ��N���ε���ɫ��ӦΪ ɫ����������ζ����Է�����ɫ��Ӧ����ԭ���� ��
��4��Ԫ��M��Ԫ��Q�γɾ���ṹ��ͼ��ʾ�����侧���߳�Ϊa pm����aλ����bλ��֮��ľ���Ϊ_______pm��ֻҪ������ʽ����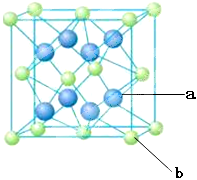
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F����Ԫ��Ϊԭ��������������Ķ�����Ԫ�ء�AΪԭ�Ӱ뾶��С��Ԫ�أ�A��B���γ�4ԭ��10���ӵķ���X��C���������������ڲ��3���� Dԭ�ӵ����������������ڲ��������һ�룻E�ǵؿ��к������Ľ���Ԫ�أ�FԪ�ص��������������۴�����Ϊ6��
��ش��������⣺
(1)B��C��D��E��F����Ԫ��ԭ�Ӱ뾶�ɴ�С��˳����________��
(2)A��C��ԭ�Ӹ�����1��l�γ�4ԭ�ӷ���Y��Y�Ľṹʽ��________��
(3)����X�ĵ���ʽ��_____________��D������Һ̬X�з�����������A2C�ķ�Ӧ��
д����Ӧ�Ļ�ѧ����ʽ_________________________________ ��
(4)ʵ��֤�������ڵ�EF3�����磬��ԭ����__________________________��
(5)E�ĵ��ʿ�����A��C��D�γɵĻ�����Z��ˮ��Һ�С���������֤ʵ�˷�Ӧ���ɵ���������Ҫ��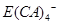 ����ʽ���ڣ�д��E����Z��Һ�����ӷ���ʽ��_________________________________________.
����ʽ���ڣ�д��E����Z��Һ�����ӷ���ʽ��_________________________________________.
(6)��ҵƷZ����Һ�к���ijЩ����������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ��ֻ����������ͨ�������乤��ԭ����ͼ��ʾ��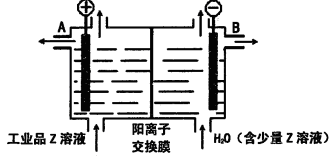
�ٸõ��۵�������Ӧʽ��____________________��
��ͨ�翪ʼ������������ҺpH_________________������������С�����䡱����
�۳�ȥ���ʺ��Z��Һ��Һ�����___________________����д��A����B����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��(As)�ڵؿ��к�������,����Ļ�����ȴ�Ƿḻ��ʡ�
��1����Ļ�̬ԭ�ӵĵ����Ų�ʽΪ ��
��2��Ŀǰ���۵ķ�������ܣ���������黯��(GaAs)Ϊ����Ga��As���,�縺�Խϴ���� ,GaAs��Ga�Ļ��ϼ�Ϊ ��
��3��AsH3����ɫ���д�����ζ�����壬��AsH3��Asԭ�ӵ��ӻ��������Ϊ ��
AsH3�ķе����PH3������Ҫԭ��Ϊ ��
��4��Na3AsO4����ɱ�����AsO43-�����幹��Ϊ �����以Ϊ�ȵ�����ķ��ӵĻ�ѧʽΪ (��дһ��)��
��5��H3AsO4��H3AsO3��������ֺ�����,����ݽṹ�����ʵĹ�ϵ������H3AsO4��H3AsO3����ǿ��ԭ�� ��
��6��������ͬ���壬��һ�ֵ��ʰ��ף�P4)���ڷ��Ӿ��壬�侧����ͼ����֪��������������Ӽ����Ϊ a pm�������ӵ�������ֵΪNA����þ�����ܶ�Ϊ______g/cm3(ֻҪ������ʽ�����ؼ��㣩��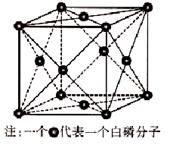
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ֶ�����Ԫ��X��Y��Z��W�����У�
�� X��Wԭ�ӵĵ��Ӳ���������������֮�ȷֱ�Ϊ3:1��1:3
�� Yԭ����Ԫ�����ڱ���ԭ�Ӱ뾶��С��
�� Z���γɻ�������������Ԫ��
�ش��������⣺
��1��XԪ�������ڱ��е�λ���������������������� ����
��2����������Ԫ����������ɵĺ��зǼ��Թ��ۼ��Ļ�����ķ���ʽ��������������������һ�ּ��ɣ���
��3��������X2W2�ĵ���ʽ�������������� ��
��4����1 mol Na2SiO3����Һ�л���ͨ��2 mol��ZO2����Ӧ�����ӷ���ʽ������������ ����
��5����Y��Z��WԪ���е����ֻ����ֿ�����ɶ��ַ��ӣ����к���10�����ӵķ�����_________���ѧʽ����
��6����25.00 mL��Y2Z2W4��Һ�м���5.00 mL 0.50 mol/L����KMnO4��Һǡ����ȫ��Ӧ���õ�ZO2��Mn2+�������Y2Z2W4��Һ�����ʵ���Ũ������������ ��mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣�Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���������������Ϣ���ɣ������ǰ��ֶ�����Ԫ�ص������Ϣ����֪���ԭ�Ӱ뾶Ϊ0.089nm��
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.16 | 0.143 | 0.102 | 0.099 | 0.074 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��15�֣�
������Ԫ��X��Y��Z��W�����ڱ��е�λ����ͼ��ʾ������W���������������γɵ���Ҫ���ʡ�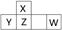
��1�� д��W��ԭ�ӽṹʾ��ͼ�� ��
��2�� ������X�����������ͨ�뺬YԪ�ص���������Һ�С���Ӧ�����ӷ���ʽΪ ��
��3�� ��֪:��X(s) + O2(g) ��XO2(g)�� ��H����393.5 kJ��mol��1
��H2(g) + 1/2 O2(g) ��H2O(g)���� ��H����242.0 kJ��mol��1
��XH4(g) + 2O2(g) ��XO2(g) + 2H2O(g) ��H����802.0 kJ��mol��1
��XH4����ֽ��������X������Ȼ�ѧ����ʽΪ�� ������
��4�� ZO���ɵ���X��ZO2��Ӧ��ȡ����Z���м�����������ʱ��ZO��NaOH��Һ��Ӧ(���ﺬ��һ�ֹ��嵥�ʺ�һ������)�Ļ�ѧ����ʽΪ_______________��
��5�� ����ԭ���ԭ��������W��һ�������O2��H2O���Ʊ�W������������Ӧˮ���д���õ�ظ�����Ӧʽ��___________��
��6�� ��W����̬�⻯��ͨ��һ������NaOH��Һ�У���������Һ����μ���ϡ����������������������HCl�����ʵ����Ĺ�ϵ��ͼ��ʾ(����������ܽ��HCl�Ļӷ�)��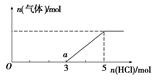
��O����Һ���������ʵĻ�ѧʽΪ____________��
��a����Һ�У�c(Na+): c(Cl��)= _______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��12�֣�A��B��C��D��E��F����Ԫ�ط������������ڣ���ԭ��������������A��Dͬ���壬���γ����ӻ�����X��B���⻯����F���⻯��ɷ�Ӧ�������ӻ�����Y�� ��B�ĵ����ǿ����к�����ߵ����ʣ�Cԭ�ӵ����������Ǵ�����������3����D��E��F 3��ԭ������㹲��11�����ӣ� ����3��Ԫ�ص�����������ˮ�����������ܷ�����Ӧ�����κ�ˮ��
��1��BԪ�ص�������________��B���ʵĽṹʽΪ_____________��
��2���õ���ʽ��ʾ������X���γɹ���__________________________________��
��3��������Y�ĵ���ʽΪ_____________��A2C2�ĵ���ʽΪ_____________��
��4��D��E����������ˮ����֮�䷴Ӧ�����ӷ���ʽΪ ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и�������У�����ԭ���ӻ���������Ͳ���ͬ����
| A��CO2��SO2 | B��CH4��NH3 | C��SO3��BF3 | D��H2S��CCl4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com