
| A����Һ����ȷ��Al3+�Ĵ������ |
| B��ԭ��Һ�в���������Ϊ��K+��Al3+��CO32- |
| C��������п���ȷ��Fe2+��NO3-�Ĵ��� |
| D��������й���2�ֱ��γ��� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
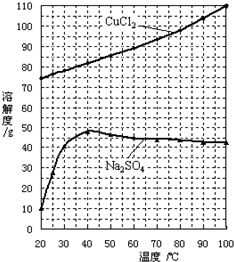
| �¶�/�� | 32 | 52 | 90 | 120 |
| ��������/g | �� | 83 | 74 | 65 |
| �������ʻ�ѧʽ | CoCl2?6H2O | �� | �� | CoCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Na��Mg��Al�����ʵ��� x mol | x��
|
| ��3�� | ��4�� | ||||
| H2����� | ��1�� | ��2�� | VNa��VMg=VAl | VNa=VMg=VAl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
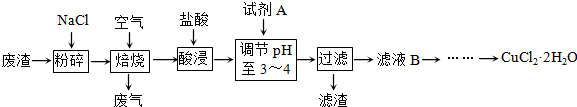
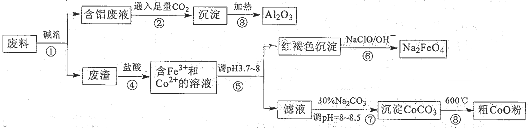
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� �ռ����� |
B�� ģ��ʯ������ |
C��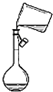 ת����Һ |
D��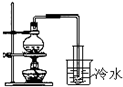 �ú�ˮ����������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������Ԫ�أ�����A��B��C��D��EΪ������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش��������⣮
��������Ԫ�أ�����A��B��C��D��EΪ������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش��������⣮| ��AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| ��Bԭ��L����2��δ�ɶԵ��� |
| ��CԪ��ԭ�ӵĺ���p��������s��������1 |
| ��DԪ�������������ǵ��Ӳ�����3�� |
| ��Eԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ�I1=738kJ/mol��I2=1451kJ/mol��I3=7733kJ/mol��I4=10540kJ/mol |
| ��F��ǰ�������е縺����С��Ԫ�� |
| ��Gԭ�Ӻ���M��ȫ������N��ֻ��1������ |
 ��ͬѧ�����ĵ����Ų�ͼΥ����
��ͬѧ�����ĵ����Ų�ͼΥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
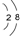
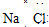
2 1 |
6 3 |
7 3 |
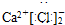
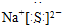
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Q2��Q1=92.4kJ |
| B��Q2=Q1=92.4kJ |
| C��Q2��Q1��92.4kJ |
| D��Q2=Q1��92.4kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
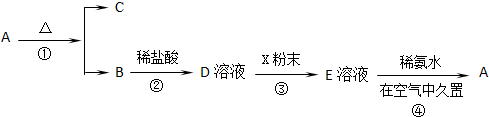
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com