��������һ�������£�2.30g����A��5.35gNH
4Cl����ǡ����ȫ��Ӧ�����ɹ���B��4.48L����C ����״����������C��������ˮ�õ�������Һ������֪CΪNH
3�������ˮB������һ�ֶ�����Ԫ�صĽ�������D��������BΪ����D�Ȼ��4.48L���������ʵ���=
=0.2mol��������=0.2mol��17g/mol=3.4g�����������غ��֪B������Ϊ2.3g+5.35g-3.4g=4.25g��NH
4Cl��Ħ������Ϊ53.5g/mol��5.35gNH
4ClΪ0.1mol����DΪ��A������������A��NH
4Cl���巴Ӧ�ɱ�Ϊ��A+NH
4Cl��DCl
2+NH
3������Clԭ���غ㣬DCl
2�����ʵ���=0.05mol����Ħ������=
=85g/mol��D����Է�������=85-71=14�����������⣬��DΪ��A������������A��NH
4Cl���巴Ӧ�ɱ�Ϊ��A+NH
4Cl��DCl+NH
3������Clԭ���غ㣬DCl�����ʵ���=0.1mol����Ħ������=
=42.5g/mol��D����Է�������=42.5-35.5=7����DΪLi������֪BΪLiCl����ô2.3g������A�к�LiԪ��ҲΪ 0.1mol���ٸ��������غ��ԭ���غ㣨ԭ�ӵ��������Ŀ��Ӧǰ����ͬ������2.3gA�к���Nԭ��Ϊ0.2mol-0.1mol=0.1mol������Hԭ��Ϊ0.2mol��4-0.4mol=0.2mol������֪A��LiNH
2��
��1��CΪ�����ǹ��ۻ�������дC�ĵ���ʽ��
��2������ԭ���غ���ƽ��д��ѧ����ʽ��
���ݻ�����A��LiNH
2����ˮǿ��ˮ�⣬������LiOH��NH
3�������������ᷴӦ����LiCl��NH
4Cl��
��3������Ԫ�ػ��ϼ۱仯������Ԫ�ػ��ϼ�����ʧȥ��������ԭ�� ����������������Ӧ����������Ӧ���ʵ��������㣻
��4������ԭ���غ���дA��750��800��ֽ�ķ���ʽ��
��5��Li
2NH��HԪ�صĻ��ϼ���+1����Ԫ�صĻ��ϼ�Ϊ-3��LiNH
2�е�Ԫ�صĻ��ϼ�Ϊ-3��HԪ�صĻ��ϼ���+1��LiH��HԪ�صĻ��ϼ���-1�����Դӻ��ϼ۱仯�ĽǶ����ж�������ԭ��Ӧ���йظ�����Ӻ�����Ӳ���Խ�࣬�뾶Խ��ƿ�������������̣�
��6��LiNH
2�ܶȴ��ڱ���ױ��Ҳ��������ǣ����Կ��ñ���ױ����и��ǣ��Ҵ��ǻ��ϵ���ϻ��ã���Ҳ���Ը�LiNH
2��Ӧ���������ڴ��ǻ��ϵ����ˮ���ⲻ���ã��ʴ˷�Ӧ���нϻ������ɽ��������ֲ�����Σ�գ�
���
�⣺��һ�������£�2.30g����A��5.35gNH
4Cl����ǡ����ȫ��Ӧ�����ɹ���B��4.48L����C ����״����������C��������ˮ�õ�������Һ������֪CΪNH
3�������ˮB������һ�ֶ�����Ԫ�صĽ�������D��������BΪ����D�Ȼ��4.48L���������ʵ���=
=0.2mol��������=0.2mol��17g/mol=3.4g�����������غ��֪B������Ϊ2.3g+5.35g-3.4g=4.25g��NH
4Cl��Ħ������Ϊ53.5g/mol��5.35gNH
4ClΪ0.1mol����DΪ��A������������A��NH
4Cl���巴Ӧ�ɱ�Ϊ��A+NH
4Cl��DCl
2+NH
3������Clԭ���غ㣬DCl
2�����ʵ���=0.05mol����Ħ������=
=85g/mol��D����Է�������=85-71=14�����������⣬��DΪ��A������������A��NH
4Cl���巴Ӧ�ɱ�Ϊ��A+NH
4Cl��DCl+NH
3������Clԭ���غ㣬DCl�����ʵ���=0.1mol����Ħ������=
=42.5g/mol��D����Է�������=42.5-35.5=7����DΪLi������֪BΪLiCl����ô2.3g������A�к�LiԪ��ҲΪ 0.1mol���ٸ��������غ��ԭ���غ㣨ԭ�ӵ��������Ŀ��Ӧǰ����ͬ������2.3gA�к���Nԭ��Ϊ0.2mol-0.1mol=0.1mol������Hԭ��Ϊ0.2mol��4-0.4mol=0.2mol������֪A��LiNH
2��
��1��CΪ�����ǹ��ۻ�������дC�ĵ���ʽΪ��
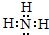
��
�ʴ�Ϊ��
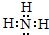
��
��2������ڴ�������ȼ�տ��Ƶ�Li
3N���䷴Ӧ�Ļ�ѧ����ʽΪ6Li+N
22Li
3N��
�ʴ�Ϊ��6Li+N
22Li
3N��
��3����Ӧ�Ļ�ѧ����ʽΪ��Li
3N+2H
2LiNH
2+2LiH����Ԫ�ػ��ϼ�0�۱仯Ϊ+1�ۺ�-1�ۣ����ϼ����ߵ�����ԭ�������������������������������ΪLiNH
2 ����270��ʱ���÷�Ӧ���������ų�H
2���������﮿���Ϊ������ϣ����ջ�ѧ����ʽ���㣬�����������ɴ�Li
3N������=
��100%=11.4%��
�ʴ�Ϊ��LiNH
2 ��11.4��
��4��A��LiNH
2����750��800��ֽ�ķ���ʽΪ��3LiNH
2Li
3N+2NH
3��
�ʴ�Ϊ��3LiNH
2Li
3N+2NH
3��
��5��A��Li
2NH�е�Ԫ�صĻ��ϼ�Ϊ-3����A����
B����Ӧ��H
2�е���Ԫ�صĻ��ϼ�Ϊ0�ۣ���Ӧ������LiNH
2��HԪ�صĻ��ϼ���+1��LiH��HԪ�صĻ��ϼ���-1������H
2�������������ǻ�ԭ������B��ȷ��
C��Li
+������һ�����Ӳ㣬H
+�������ӣ����Ӻ�����Ӳ���Խ�࣬�뾶Խ��Li
+�뾶����H
+����C����
D����ƿ�������������̣����÷���Ϊ��ѧ��������D����
��ѡB��
�ʴ�Ϊ��B��
��6�����õ�A���ֱܴ��ʶ�����ʹ�ã���Ҫ�������٣�����������������ñ���ױ����串�ǣ�Ȼ���������ñ���ױ�ϡ������ˮ�Ҵ���LiNH
2�ܶȴ��ڱ���ױ��Ҳ��������ǣ����Կ��ñ���ױ����и��ǣ��Ҵ��ǻ��ϵ���ϻ��ã���Ҳ���Ը�LiNH
2��Ӧ������ʽΪLiNH
2+C
2H
5OH-��C
2H
5OLi+NH
3���������ڴ��ǻ��ϵ����ˮ���ⲻ���ã��ʴ˷�Ӧ���нϻ������ɽ��������ֲ�����Σ�գ�
�ʴ�Ϊ��LiNH
2�ܶȴ��ڱ���ױ��Ҳ��������ǣ����Կ��ñ���ױ����и��ǣ��Ҵ��ǻ��ϵ���ϻ��ã���Ҳ���Ը�LiNH
2��Ӧ������ʽΪLiNH
2+C
2H
5OH-��C
2H
5OLi+NH
3���������ڴ��ǻ��ϵ���û��ˮ������ã��ʴ˷�Ӧ���нϻ������ɽ��������ֲ�����Σ�գ�

 ��
�� ��
��


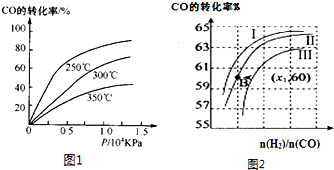
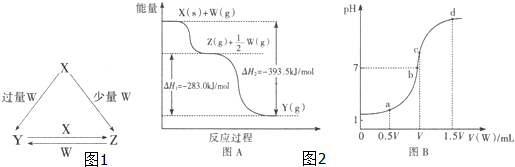
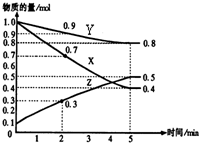 ��1��ij�¶�ʱ����2L�ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��仯������ͼ��ʾ����ͼ�����ݷ������÷�Ӧ�Ļ�ѧ����ʽΪ��
��1��ij�¶�ʱ����2L�ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��仯������ͼ��ʾ����ͼ�����ݷ������÷�Ӧ�Ļ�ѧ����ʽΪ��