
���� ij��ϩ��A��������Ϣ�������·�����Ӧ���õ����ֲ���B��C����B��CӦΪȩ��ͪ������B�ܷ���������Ӧ��C���ܣ���˵��B����ȩ����CΪͪ��B��C�Ļ�ѧʽ��ΪC3H6O����BΪCH3CH2CHO��CΪCH3COCH3�����������Ϣ����֪AΪ��CH3��2C�TCHCH2CH3���ݴ˴��⣮
��� �⣺ij��ϩ��A��������Ϣ�������·�����Ӧ���õ����ֲ���B��C����B��CӦΪȩ��ͪ������B�ܷ���������Ӧ��C���ܣ���˵��B����ȩ����CΪͪ��B��C�Ļ�ѧʽ��ΪC3H6O����BΪCH3CH2CHO��CΪCH3COCH3�����������Ϣ����֪AΪ��CH3��2C�TCHCH2CH3��
�ʴ�Ϊ����CH3��2C�TCHCH2CH3��CH3CH2CHO��CH3COCH3��
���� ���⿼���л�����ƶϣ��ѶȲ����� �Ĺؼ��dz������������Ϣ����Ϸ���ʽ�����ƶϣ�ע�ض��л�����֪ʶ�Ŀ��飬ͬʱ����ѧ��Ӧ����Ϣ�Լ���ϢǨ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
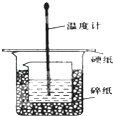 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ���� ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2��/�� | �²� ��t2-t1��/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuSO4 •5H2O�����������ﶼ�Ǵ����� | |
| B�� | һ�����ʲ��ǵ���ʾ��Ƿǵ���� | |
| C�� | ϡ���ᡢNaCl��Һ��ʵ���ҳ����ĵ���� | |
| D�� | ��������ɹ㷺����ʳƷ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 2��3��1 | B�� | 1��2��3 | C�� | 4��3��2 | D�� | 3��2��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4 | B�� | 6 | C�� | 8 | D�� | 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Al��OH��3 | Ga��OH��3 | |
| ��ʽ���볣��Ka | 2��10-11 | 1��10-7 |
| ��ʽ���볣��Kb | 1.3��10-33 | 1.4��10-34 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| N2��g�� | + | 3H2��g�� | ? | 2NH3 | |
| �� | 1mol | 3mol | 0mol | ||
| �� | 0mol | 0mol | 2mol | ||
| �� | 0.5mol | 1.5mol | 1mol | ||
| �� | 1mol | 3mol | 2mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Na2CO3���� | B�� | CH3COONa���� | C�� | Na2SO4��Һ | D�� | NaNO3��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com