���㣺�����ļ���,ԭ�Ӻ�������Ų�,Ԫ�ص����ܡ��縺�Եĺ��弰Ӧ��,�жϼ��ӻ����ӵĹ���,���ȵ���ԭ������Ӧ��
ר�⣺ԭ�������ṹר��,��ѧ���뾧��ṹ
��������1���ٵ�ԭ�ӵ�L���ϣ�2s�ܼ�����2�����ӡ�2p�ܼ�����3�����ӣ������������ԭ�������ع����ﲻ����ԭ����д��
��ͬһ�����У�Ԫ�ص�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�ش�������Ԫ�أ�
�ۼ۵�������ȡ�ԭ�Ӹ�����ȵ�������ȵ����壻
�ܰ��������е�ԭ�Ӻ�ÿ����ԭ��֮�䶼����һ�����õ��Ӷԣ�
��2������Ĵ��ڵ������ʵ��۷е����ߣ�
��3�����ݼ۲���ӶԻ�������ȷ��NO
-3�乹�ͣ�
��4��A�����������ɽ��������Ӻ����ɵ��ӹ��ɵģ�
B����-Fe����-Fe�Ķѻ���ʽ�ֱ���غ�ͭ��ͬ��
C���ռ������ʦ�-FeС�ڦ�-Fe��
D�����������д��ڽ�������
��5����-Fe��Feԭ�Ӹ���=1+8��
=2����-Fe��Feԭ�Ӹ���=8��
+6��=4����Feԭ�Ӱ뾶Ϊr����-Fe�о����߳�=
����-Fe�о����ı߳�=
��
��=��
���
�⣺��1���ٵ�ԭ�ӵ�L���ϣ�2s�ܼ�����2�����ӡ�2p�ܼ�����3�����ӣ������������ԭ�������ع����ﲻ����ԭ��֪����L������Ų�ͼΪ
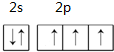
���ʴ�Ϊ��

��
��ͬһ�����У�Ԫ�ص�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�ش�������Ԫ�أ����Եڶ�����Ԫ���е�һ������������Ne���ʴ�Ϊ��Ne��
�ۼ۵�������ȡ�ԭ�Ӹ�����ȵ�������ȵ����壬��N
3��Ϊ�ȵ������������CO
2�ȣ��ʴ�Ϊ��CO
2�ȣ�
�ܰ��������е�ԭ�Ӻ�ÿ����ԭ��֮�䶼����һ�����õ��Ӷԣ��������ӵĵ���ʽΪ
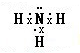
���ʴ�Ϊ��
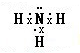
��
��2��NH
3��������N
2O�в��������������Ĵ��ڵ���NH
3�е㣨-33.34�棩��N
2O�е㣨-88.49�棩�ߣ��ʴ�Ϊ���������Ӽ���������
��3��NO
-3�м۲���ӶԸ���=3+
��5+1-3��2��=3�Ҳ����µ��Ӷԣ�������ռ乹��Ϊƽ�������Σ��ʴ�Ϊ��ƽ�����ǣ�
��4��A���������ĵ�����������ͨ��ʱ���ɵ����������ƶ������Ӳ���ͨ��Ų����ģ��ʴ���
B����-Fe����-Fe�Ķѻ���ʽ�ֱ���غ�ͭ��ͬ����-FeΪ���������ѻ�����-FeΪ���������ѻ�������ȷ��
C���ռ������ʦ�-FeС�ڦ�-Fe���ʴ���
D�����������д��ڽ����������Խ������ڲ����ڽ�����������ȷ��
��ѡBD��
��5����-Fe��Feԭ�Ӹ���=1+8��
=2����-Fe��Feԭ�Ӹ���=8��
+6��=4����Feԭ�Ӱ뾶Ϊr����-Fe�о����߳�=
����-Fe�о����ı߳�=
����-Fe�ܶ�
��==
����-Fe�ܶ�=
�����ܶ�֮��=
��
=0.92��
�ʴ�Ϊ��0.92��
���������⿼�������ʽṹ�����ʣ��漰�������㡢�ȵ����塢���ӿռ乹�͵��жϵ�֪ʶ�㣬��Щ֪ʶ�㶼�Ǹ߿��ȵ㣬�������û���֪ʶ������⣬�ѵ��Ǿ����ļ��㣬ע�⣺��-Fe����-Fe���侧���߳�����Feԭ��ֱ������-Fe����������Խ��߱߳�Ϊ4��ԭ�Ӱ뾶����-Fe��ÿ�����϶Խ��߱߳�Ϊ4��Feԭ�Ӱ뾶���˵�Ϊ�״��㣮

 �������ƣ�NaN3����һ����ɫ���壬�㷺����������ȫ���Ҽ������ϳɵȣ������������Ʊ�����Ϊ��
�������ƣ�NaN3����һ����ɫ���壬�㷺����������ȫ���Ҽ������ϳɵȣ������������Ʊ�����Ϊ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��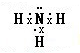 ���ʴ�Ϊ��
���ʴ�Ϊ��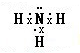 ��
��

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
 ������ͼװ�õ���������ȷ���ǣ�������
������ͼװ�õ���������ȷ���ǣ�������



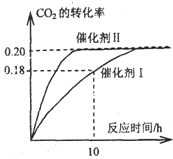 I����֪CO2����������ɫȼ�ϼ״���CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H=-87.4kJ?mol-1��300��ʱ����һ���ݻ����ܱ������У���c��CO2��=1.00kJ?mol-1��c��H2��=1.60kJ?mol-1��ʼ��Ӧ�������ͼ��ʾ���ش��������⣺
I����֪CO2����������ɫȼ�ϼ״���CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H=-87.4kJ?mol-1��300��ʱ����һ���ݻ����ܱ������У���c��CO2��=1.00kJ?mol-1��c��H2��=1.60kJ?mol-1��ʼ��Ӧ�������ͼ��ʾ���ش��������⣺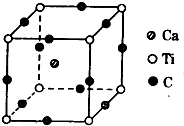 ������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2np2��CԪ��λ�ڵڶ�������ԭ����p�Dz�������s�Dz����������ȣ�DԪ��ԭ�ӵ�L���p�Dz�����3��δ�ɶԵ��ӣ�
������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2np2��CԪ��λ�ڵڶ�������ԭ����p�Dz�������s�Dz����������ȣ�DԪ��ԭ�ӵ�L���p�Dz�����3��δ�ɶԵ��ӣ�