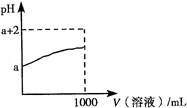
��ͼ����Emil Zmaczynski��ƵĽ�����ʽԪ�����ڱ���һ���֣�ͼ�ϱ��еڢ�A��ͼ���Ԫ�ص�λ�ã���ش��������⣺
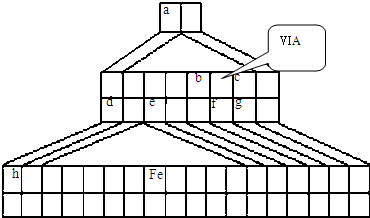
��1����ͼ�н���Ԫ�������ڱ��е�λ����
��4���ڵڢ���
��4���ڵڢ���
��2���Դ����ڱ�����������������Ԫ�������ڱ��е�λ�ã�һֱ���ڷ������������Ű������ڵڢ�A�壬�����ǣ�����������ӡ�����ȱһ������д��NaH�ĵ���ʽ
Na+[��H]-
Na+[��H]-
��3��bԪ�ص��⻯����������������ˮ���������һ���Σ���������ˮʱ
�ٽ�
�ٽ�
�������ơ������ٽ�����Ӱ�족��ˮ���룬�䷴Ӧ�����ӷ���ʽΪ
NH
4++H
2O
NH
3?H
2O+H
+NH
4++H
2O
NH
3?H
2O+H
+��
��4������������ȷ����
A��D��E
A��D��E
��
A��h������������Ӧ��ˮ������һ��ǿ��
B��f���⻯�����������ȶ�����
C��c���⻯���ˮ��Һ��ǿ��
D��ԭ�Ӱ뾶��h��e��a
E����ͬ�����£�d��f�γɵĻ�����ˮ��Һ��pH����d��g�γɵĻ�����ˮ��Һ��pH��˵����ͬŨ���⻯��ˮ��Һ������f����g
��5��d��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ��������ɫ�������Ȼ����������ʵ���֮��Ϊ1��2�������������ɣ���÷�Ӧ�����ӷ���ʽΪ��
3Na2O2+6Fe2++6H2O=4Fe��OH��3��+6Na++2Fe3+
3Na2O2+6Fe2++6H2O=4Fe��OH��3��+6Na++2Fe3+
��



 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д�
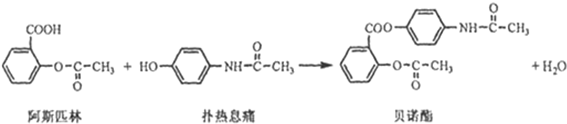
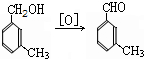
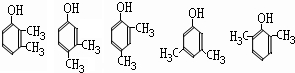
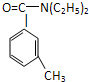 ���ð�������DEET����һ�ֶ��˰�ȫ�����Ը�����ҩ�Ե��������ü�����ṹ��ʽΪ����֪��RCOOH
���ð�������DEET����һ�ֶ��˰�ȫ�����Ը�����ҩ�Ե��������ü�����ṹ��ʽΪ����֪��RCOOH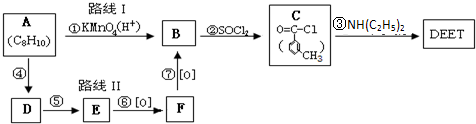
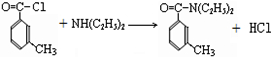
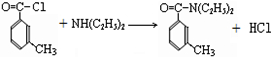

 ����д2�֣�
����д2�֣� ����д2�֣�
����д2�֣�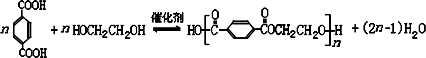


 NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+ ��2010?��������ģ��X��Y��Z��W��Ϊ����10���ӵ���������X��Y��ZΪ���ӣ�WΪ���ӣ���X��Z�����к��еĹ��õ��Ӷ���֮��Ϊ3��4��
��2010?��������ģ��X��Y��Z��W��Ϊ����10���ӵ���������X��Y��ZΪ���ӣ�WΪ���ӣ���X��Z�����к��еĹ��õ��Ӷ���֮��Ϊ3��4��