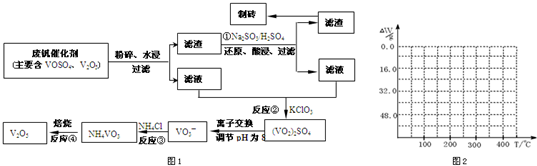
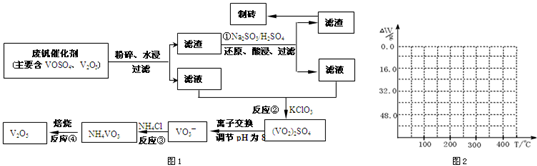
���𰸡�
��������1��
23VԪ�ص�������Ϊ23�����Ը������ڱ���λ�úͽṹ����Ԫ��λ�ڵ������ڣ��ڢ�B���壬��ҵ����V
2O
5ұ���������������ȼ������������ȷ�Ӧ��ԭ����д��ұ�����Ļ�ѧ����ʽ 3V
2O
5+10A

l6V+5Al
2O
3��
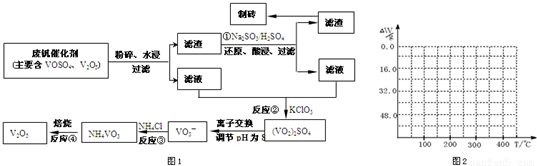
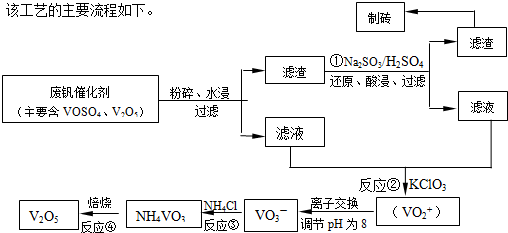
��2�������������ͼ��֪��Ӧ�ٵ�Ŀ�ģ���V
2O
5 ת��Ϊ�����Ե�VOSO
4�����ڷ����ᴿ��
��3��������֪Ũ�ȵ��ữH
2C
2O
4��Һ�ζ���VO
2��
2SO
4��Һ����Ҫ����ΪCO
2��VOSO
4��������Ӧ�����ӷ��̸���������ԭ��Ӧ�Ļ��ϼ�����������۵ķ�Ԫ�ر���ԭΪ���ļ۵ķ�Ԫ�أ�H
2C
2O
4������Ϊ������̼��д��������ƽ�ɵ����ӷ��̣�
��4����֪NH
4VO
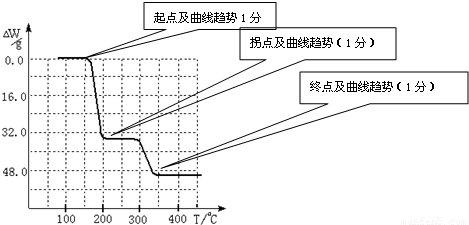
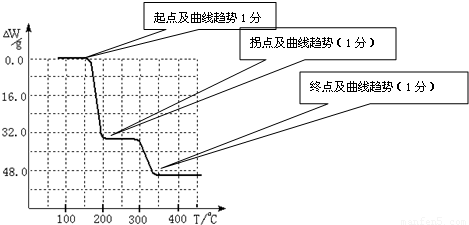
3�ڱ��չ�����150��200��ʱ��ʧȥ������300��350����ʧȥˮ�����ݷ����ķ�Ӧ�����������ˮ��������
2NH
4VO
3�TV
2O
5+2NH
3��+H
2O
234g 34g 18g
����ֵ��ʼΪ0��34g�����ߴ�150�濪ʼ���٣�34gNH
3������200��ʱ����32.0g�����������߿�ʼƽֱ����300��ʱ�ֿ�ʼ���٣�H
2O������������350��ʱ����52gʱ����48.0g�Եͣ��Ͳ��ٱ仯��������Ӧ���������������¶ȱ仯�����ߣ�
����⣺��1������
23V��������������Ϊ23��ԭ�ӣ����Է�Ԫ��λ�ڵ������ڣ��� VB ���壻������ȷ�Ӧ��ԭ��д����ѧ����ʽ���ʴ𰸣��ģ�VB�� 3V
2O
5+10A

l6V+5Al
2O
3��
��2������������������ƣ�Ŀ��������������ԭ��Ӧ�����������ƻ�ԭV
2O
5����V
2O
5 ת��Ϊ�����Ե�VOSO
4�������ᴿ���ʴ𰸣���V
2O
5 ת��Ϊ�����Ե�VOSO
4��
��3���ⶨ��Ӧ����Һ�з��ĺ�����������֪Ũ�ȵ��ữH
2C
2O
4��Һ�ζ���VO
2��
2SO
4��Һ����Ҫ����ΪCO
2��VOSO
4�����ݻ��ϼ�������д�����ӷ���Ϊ2VO
2++H
2C
2O
4+2H
+=2 VO
2++2 CO
2��+2 H
2O��
�ʴ𰸣�2VO
2++H
2C
2O
4+2H
+=2 VO
2++2 CO
2��+2 H
2O��
��4����ʽΪ��
2NH
4VO
3�TV
2O
5+2NH
3��+H
2O
234g 34g 18g
����ֵ��ʼΪ0��34g�����ߴ�150�濪ʼ���٣�34gNH
3������200��ʱ����32.0g�����������߿�ʼƽֱ����300��ʱ�ֿ�ʼ���٣�H
2O������������350��ʱ����52gʱ����48.0g�Եͣ��Ͳ��ٱ仯������ͼ������
�ʴ�Ϊ��
 ������
������������Ҫ������ԭ�ӷ��ŵĺ��塢���ȷ�Ӧ��Ӧ�á�������ԭ��Ӧ��ʵ�ʡ����ӷ��̵���д���ص㿼������ͼ��������Ӧ�й��������仯���¶ȱ仯�������軭��

 l6V+5Al2O3��
l6V+5Al2O3�� l6V+5Al2O3��
l6V+5Al2O3��



 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�

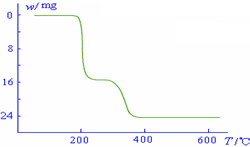 ������������ͼ��ʾ����NH4VO3�ڷֽ������
������������ͼ��ʾ����NH4VO3�ڷֽ������