
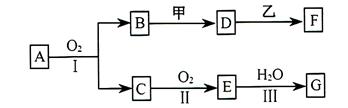
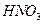 �ữ��
�ữ��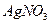 ��Һ�а�ɫ�������ɡ���
��Һ�а�ɫ�������ɡ���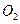 ��Ӧ������̬��B��Cʱ�ų�22.67kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��___________________________________��
��Ӧ������̬��B��Cʱ�ų�22.67kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��___________________________________�� ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
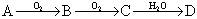 ;��֪DΪǿ�ᣬ��ش�
;��֪DΪǿ�ᣬ��ش� ����ɫ���壬B���д̼�����ζ����ɫ���塣
����ɫ���壬B���д̼�����ζ����ɫ���塣�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������Ԫ�ص�ԭ�Ӱ뾶��СΪW<X<Y<Z |
| B��W��X��Y��Zԭ�ӵĺ����������������ܺ�Ϊ20 |
| C��W��Y���γɼȺ����Թ��ۼ��ֺ��Ǽ��Թ��ۼ��Ļ����� |
| D����W��X��ɵĻ�����ķе��ܵ�����W��Y��ɵĻ�����ķе� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
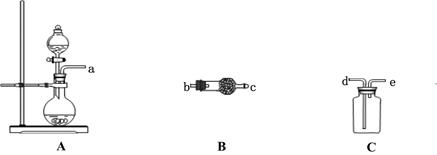
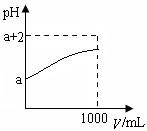
| A��Y����ˮ�ַ������� |
| B��Y��ˮ��Һ�еμ�ʯ����Һ���� |
| C��ϡ�ͺ���Һ���������ӵ�Ũ�Ⱦ���С |
| D��ϡ�ͺ������ӵ����ʵ���Ũ��֮�Ͳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
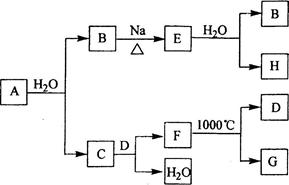
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
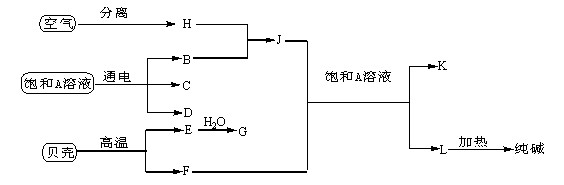

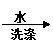
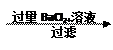
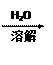
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
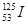 ���������������ú�����������( )
���������������ú�����������( )| A��19 | B��53 | C��72 | D��125 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
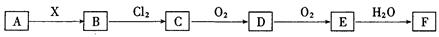
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Fe3 04 | B��F2 | C��A1203 | D��C |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com