
(8·Ö)(1)2003Äź3ŌĀČÕ±¾Öž²Ø²ÄĮĻæĘѧ¹ś¼ŅŹµŃéŹŅŅ»øöŃŠ¾æŠ”×é·¢Ļ֏ץż“ų½į¾§Ė®µÄ¾§ĢåŌŚ5KĻĀ³ŹĻÖ³¬µ¼ŠŌ”£¾Ż±ØµĄ£¬øĆ¾§ĢåµÄ»ÆѧŹ½ĪŖ Na0.35CoO2 • 1.3H2O”£ŹŌ¼ĘĖć£ŗøĆ¾§ĢåÖŠīÜŌ×ÓÓėĒāŌ×ÓµÄĪļÖŹµÄĮæÖ®±ČŹĒ___”ų_____£» 1moløĆ¾§ĢåÖŠŗ¬ÓŠµÄŃõŌ×ÓŹżÄæŹĒ____”ų_______”£
(2)ÓŠÄĘ”¢Ēā”¢Ńõ”¢ĮņĖÄÖÖŌŖĖŲ£¬ÓĆĘäÖŠµÄŅ»ÖÖ»ņ¼øÖÖŌŖĖŲæÉŅŌ×é³É¶ąÖÖĪļÖŹ£¬Š“³ö·ūŗĻĻĀĮŠŅŖĒóµÄ»Æѧ·½³ĢŹ½£ŗ
¢ŁĖįŠŌŃõ»ÆĪļŗĶ¼ī·“Ó¦£ŗ”””””””ų””””””£»””””””””””””””””””””””””
¢Ś¼īŠŌŃõ»ÆĪļŗĶĖį·“Ó¦£ŗ”””””””ų”””” ”£””
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ĘųĢåĆū³Ę | °±Ęų | ŃõĘų | ŗ¤Ęų | ÄŹĘų | ė²Ęų | ė“Ęų | ė°Ęų |
| ·Šµć/”ę | -196 | -183 | -269 | -264 | -186 | -153 | -108 |
| ŌĮĻ | ĢģČ»Ęų | ÖŲÓĶ | Ćŗ |
| Ļą¶ŌĶ¶×Ź·ŃÓĆ | 1.0 | 1.5 | 2.0 |
| ÄÜĮæĻūŗÄ/J?t-1 | 28”Į109 | 38”Į109 | 48”Į109 |
| ||
| øßĪĀ |
| ||
| øßĪĀ |
| NH3ŗ¬Įæ% Ń¹Ēæ/MPa ĪĀ¶Č/”ę |
0.1 | 10 | 20 | 30 | 60 | 100 |
| 200 | 15.3 | 81.5 | 86.4 | 89.9 | 95.4 | 98.8 |
| 300 | 2.2 | 52.0 | 64.2 | 71.0 | 84.2 | 92.6 |
| 400 | 0.4 | 25.1 | 38.2 | 47.0 | 65.2 | 79.8 |
| 500 | 0.1 | 10.6 | 19.1 | 26.4 | 42.2 | 57.5 |
| 600 | 0.05 | 4.5 | 9.1 | 13.8 | 23.1 | 31.4 |
 2NH3µÄ”÷H
2NH3µÄ”÷H| c2(NH3) |
| c(N2)?c3(H2) |
| c2(NH3) |
| c(N2)?c3(H2) |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø¹²8·Ö£©
0.80gCuSO4”¤5H2OѳʷŹÜČČĶŃĖ®¹ż³ĢµÄČČÖŲĒśĻߣØѳʷ֏ĮæĖęĪĀ¶Č±ä»ÆµÄĒśĻߣ©ČēĻĀĶ¼ĖłŹ¾”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹŌČ·¶Ø200”ꏱ¹ĢĢåĪļÖŹµÄ»ÆѧŹ½______________£ØŅŖĒ󊓳öĶʶĻ¹ż³Ģ£©
£Ø2£©Č”270”ęĖłµĆѳʷ£¬ÓŚ570”ę×ĘÉÕµĆµ½µÄÖ÷ŅŖ²śĪļŹĒŗŚÉ«·ŪÄ©ŗĶŅ»ÖÖŃõ»ÆŠŌĘųĢ壬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________”£°ŃøĆŗŚÉ«·ŪÄ©ČܽāÓŚĻ”ĮņĖįÖŠ£¬¾ÅØĖõ”¢ĄäČ“£¬ÓŠ¾§ĢåĪö³ö£¬øĆ¾§ĢåµÄ»ÆѧŹ½ĪŖ_________£¬Ęä“ęŌŚµÄ×īøßĪĀ¶ČŹĒ_____________£»
£Ø3£©ÉĻŹöŃõ»ÆŠŌĘųĢåÓėĖ®·“Ӧɜ³ÉŅ»ÖÖ»ÆŗĻĪļ£¬øĆ»ÆŗĻĪļµÄÅØČÜŅŗÓėCuŌŚ¼ÓČČŹ±·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________£»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(8·Ö) ŅŃÖŖÓŠ»śĪļAµÄĻą¶Ō·Ö×ÓÖŹĮæ²»³¬¹ż200”£Č”1.48 g AĶźČ«Č¼ÉÕŗ󣬽«Č¼ÉÕ²śĪļĶعż¼īŹÆ»Ņ£¬¼īŹÆ»ŅµÄÖŹĮæŌö¼Ó2.12g£»Čō½«Č¼ÉÕ²śĪļĶعżÅØĮņĖį£¬ÅØĮņĖįµÄÖŹĮæŌö¼Ó0.36 g£»Č”1.48 g AÓė×ćĮæÄĘ·“Ó¦£¬Éś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ0.336L”£
(1) 1.48 g AĶźČ«Č¼ÉÕÉś³ÉµÄCO2µÄĪļÖŹµÄĮæĪŖ________mol”£
(2) AµÄ·Ö×ÓŹ½ĪŖ_______________”£
(3) AÄÜŹ¹×ĻÉ«ŹÆČļŹŌŅŗ±äŗģ£¬ĒŅA¾“ß»ÆŃõ»Æŗó·Ö×ÓÖŠÖ»ÓŠŅ»ÖÖĒāŌ×Ó£¬ŌņAµÄ½į¹¹¼ņŹ½ĪŖ_____________”£
(4) ½«a g AĶźČ«Č¼ÉÕŗóµÄ²śĪļČ«²æĶØČė×ćĮæµÄNa2O2¹ĢĢå³ä·ÖĪüŹÕ£¬Ōņ¹ĢĢåÖŹĮæŌö¼Ó
____________gӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŗž±±Ź”ĪäŗŗŹŠĖÄŠ£øßČż10ŌĀĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
(8·Ö)ÖŠ¹śÕžø®³ŠÅµ£¬µ½2020Äź£¬µ„Ī»GDP¶žŃõ»ÆĢ¼ÅŷűČ2005ÄźĻĀ½µ40%~50%”£
£Ø1£©ÓŠŠ§”°¼õĢ¼”±µÄŹÖ¶ĪÖ®Ņ»ŹĒ½ŚÄÜ£¬ĻĀĮŠÖĘĒā·½·Ø×ī½ŚÄܵďĒ
A£®µē½āĖ®ÖĘĒā£ŗ2H2O 2H2”ü£«O2”ü
2H2”ü£«O2”ü
B£®øßĪĀŹ¹Ė®·Ö½āÖĘĒā£ŗ2H2O 2H2”ü£«O2”ü
2H2”ü£«O2”ü
C£®Ģ«Ńō¹ā“߻ƷֽāĖ®ÖĘĒā£ŗ2H2O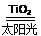 2H2”ü£«O2”ü
2H2”ü£«O2”ü
D£®ĢģČ»ĘųÖĘĒā£ŗCH4£«H2O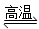 CO£«3H2
CO£«3H2
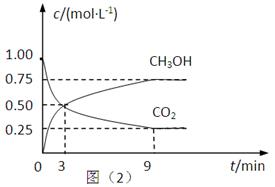
£Ø2£©CO2æÉ×Ŗ»Æ³ÉÓŠ»śĪļŹµĻÖĢ¼Ń»·”£ŌŚĢå»żĪŖ1LµÄĆܱÕČŻĘ÷ÖŠ£¬³äČė1mol CO2ŗĶ3mol H2£¬Ņ»¶ØĢõ¼žĻĀ·“Ó¦£ŗCO2(g)+3H2(g) CH3OH(g)+H2O(g) ”÷H=£49.0kJ”¤mol£1£¬²āµĆCO2ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆČēÉĻĶ¼ĖłŹ¾”£
CH3OH(g)+H2O(g) ”÷H=£49.0kJ”¤mol£1£¬²āµĆCO2ŗĶCH3OH(g)µÄÅضČĖꏱ¼ä±ä»ÆČēÉĻĶ¼ĖłŹ¾”£
¢Ł“Ó3 minµ½9 min£¬v(H2)=________mol”¤L£1”¤min£1”£
¢ŚÄÜĖµĆ÷ÉĻŹö·“Ó¦“ļµ½Ę½ŗāדĢ¬µÄŹĒ____________£ØĢī±ąŗÅ£©”£
A£®·“Ó¦ÖŠCO2ÓėCH3OHµÄĪļÖŹµÄĮæÅضČÖ®±ČĪŖ1”Ć1£Ø¼“Ķ¼ÖŠ½»²ęµć£©
B£®»ģŗĻĘųĢåµÄĆÜ¶Č²»Ėꏱ¼äµÄ±ä»Æ¶ų±ä»Æ
C£®µ„Ī»Ź±¼äÄŚĻūŗÄ3mol H2£¬Ķ¬Ź±Éś³É1mol H2O
D£®CO2µÄĢå»ż·ÖŹżŌŚ»ģŗĻĘųĢåÖŠ±£³Ö²»±ä
£Ø3£©¹¤ŅµÉĻ£¬CH3OHŅ²æÉÓÉCOŗĶH2ŗĻ³É”£²Īæ¼ŗĻ³É·“Ó¦CO(g)+2H2(g) CH3OH(g)µÄĘ½ŗā³£Źż£ŗ
CH3OH(g)µÄĘ½ŗā³£Źż£ŗ
|
ĪĀ¶Č/”ę |
0 |
100 |
200 |
300 |
400 |
|
Ę½ŗā³£Źż |
667 |
13 |
1.9”Į10-2 |
2.4”Į10-4 |
1”Į10-5 |
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ_____”£
A£®øĆ·“Ó¦Õż·“Ó¦ŹĒ·ÅČČ·“Ó¦
B£®øĆ·“Ó¦ŌŚµĶĪĀĻĀ²»ÄÜ×Ō·¢½ųŠŠ£¬øßĪĀĻĀæÉ×Ō·¢½ųŠŠ£¬ĖµĆ÷øĆ·“Ó¦”÷S£¼0
C£®ŌŚT”ꏱ£¬1LĆܱÕČŻĘ÷ÖŠ£¬Ķ¶Čė0.1mol COŗĶ0.2 mol H2£¬“ļµ½Ę½ŗāŹ±£¬CO×Ŗ»ÆĀŹĪŖ50%£¬Ōņ“ĖŹ±µÄĘ½ŗā³£ŹżĪŖ100
D£®¹¤ŅµÉĻ²ÉÓĆÉŌøßµÄŃ¹Ēæ(5Mpa)ŗĶ250”ę£¬ŹĒŅņĪŖ“ĖĢõ¼žĻĀ£¬ŌĮĻĘų×Ŗ»ÆĀŹ×īøß
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗÓ±±Ź”øßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
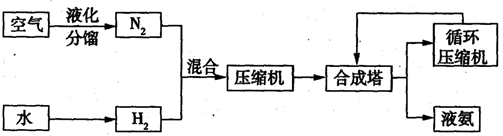
(8·Ö)ĻĀ±ķ±ķŹ¾ŗĻ³É°±·“Ó¦£ØN2+3H2  2NH3£©ŌŚ²»Ķ¬Ģõ¼žĻĀ“ļµ½Ę½ŗāŹ±»ģŗĻĪļÖŠ°±µÄŗ¬Įæ[ĘšŹ¼Ź±v£ØN2£©£ŗv£ØH2£©==1£ŗ3]”£
2NH3£©ŌŚ²»Ķ¬Ģõ¼žĻĀ“ļµ½Ę½ŗāŹ±»ģŗĻĪļÖŠ°±µÄŗ¬Įæ[ĘšŹ¼Ź±v£ØN2£©£ŗv£ØH2£©==1£ŗ3]”£
|
ĪĀ¶Č£Ø”ę£© |
0.1 |
10 |
30 |
60 |
100 |
|
200 |
0.153 |
0.815 |
0.899 |
0.954 |
0.988 |
|
300 |
0.022 |
0.520 |
0.710 |
0.842 |
0.926 |
|
400 |
0.004 |
0.251 |
0.470 |
0.652 |
0.798 |
·ÖĪöÉĻ±ķŹż¾Ż,»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©200”ę”¢100MPaŹ±£¬Ę½ŗā»ģŗĻĪļÖŠ°±µÄŗ¬ĮæŅŃ“ļ0.988£¬Čē¹ū¼ĢŠųŌö“óŃ¹Ēæ
£ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©Ź¹Ę½ŗā»ģŗĻĪļÖŠ°±µÄŗ¬ĮæµČÓŚ1£¬ĄķÓÉŹĒ£ŗ
ӣ
£Ø2£©ÓūŹ¹Ę½ŗā»ģŗĻĪļÖŠ°±µÄŗ¬ĮæŌö“ó£¬ŌņæɲÉČ”µÄ“ėŹ©ÓŠ£ŗ ”£
£Ø3£© ÓūŹ¹Ę½ŗā»ģŗĻĪļÖŠ°±µÄŗ¬ĮæĪŖ0.710£¬ŌņŃ”ŌńµÄ·“Ó¦Ģõ¼žÓ¦ĪŖ£ŗ
ÓūŹ¹Ę½ŗā»ģŗĻĪļÖŠ°±µÄŗ¬ĮæĪŖ0.710£¬ŌņŃ”ŌńµÄ·“Ó¦Ģõ¼žÓ¦ĪŖ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com