
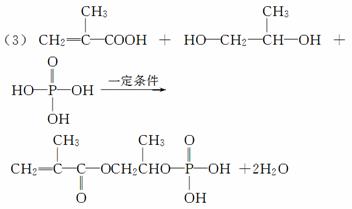
�߷��Ӳ���M�ڹ�ۺ���ͽ���Ϳ�Ϸ�������Ҫ��;��M�Ľṹ��ʽΪ
COCCH2CH3OCH2CHCH3OPOHOOH
��ҵ�Ϻϳ�M�Ĺ��̿ɱ�ʾ���£�
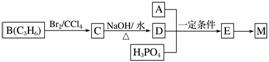
��֪��A��ȫȼ��ֻ����CO2��H2O���������ܶ�����ͬ״���������ܶȵ�43����������H��Oԭ�Ӹ�����Ϊ3��1������Na��Na2CO3���ܷ�Ӧ������ɫ���塣
(1)A�����������ŵ�������____________��
(2)����˵����ȷ����________(����ĸ)��
a����ҵ�ϣ�B��Ҫͨ��ʯ�ͷ�����
b��C��ͬ���칹����3��(������C)
c��E����M�ķ�Ӧ�����۷�Ӧ
(3)д��A��D��H3PO4����E��Ӧ�Ļ�ѧ����ʽ��_________________________________��
�÷�Ӧ������________��Ӧ��
(4)F��A��һ��ͬ���칹�壬F�ĺ˴Ź���������ʾ�����������ֲ�ͬ����ԭ�ӡ���������ת����ϵ��
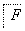
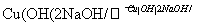
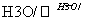
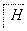
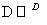
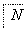
д��F��N�Ľṹ��ʽF��__________________________________________��
N��________________________________________________________________________��
д����Ӧ�ٵĻ�ѧ����ʽ____________________________________________________
________________________________________________________________________��
�𰸡�(1)̼̼˫�����Ȼ���(2)b
����(��ȡ��)
(4)OHCCH2CH2CHO

OHC—CH2—CH2—CHO��4Cu(OH)2��2NaOH��,NaOOC—CH2—CH2—COONa��2Cu2O����6H2O
����������A�������ܶ�����ͬ״���������ܶȵ�43��������ȷ��A����Է�������Ϊ86��������ʷ���ʽΪCxH3yOy��������Է��������������x��4��y��2��A�ķ���ʽΪC4H6O2�����ݺϳ�M����ͼ�������ɵ�B�Ľṹ��ʽΪCHCH3CH2��C�Ľṹ��ʽΪ
CH(Br)CH3CH2(Br)��D�Ľṹ��ʽΪ
CH(OH)CH3CH2(OH)��E�Ľṹ��ʽΪ
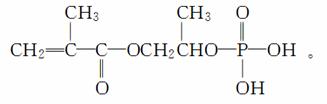
����֪��֪��F����ȩ��������H�������D��Ӧ���ɸ߷��ӻ����˵��H�к��������Ȼ�����F��CH2OHCCH2CHO��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
20 g AO32-�к������������������0.5NA(NA���������ӵ�����)������Ԫ��A�����ԭ������Ϊ( )
A.12 B.32 C.60 D.80
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС���Ա�����ϩΪ��Ҫԭ�ϣ���������·�ߺϳ�ҩ����³����

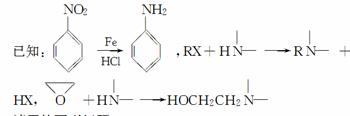
��ش��������⣺
(1)������³��������˵����ȷ����________��
A������Ũ�����γ���
B���������������ӳɷ�Ӧ
C���ɷ���ˮ�ⷴӦ
D���������
(2)д��������B�Ľṹ��ʽ________________��
(3)д��B����C��Ӧ������Լ�____________��
(4)д��C��D����E�Ļ�ѧ��Ӧ����ʽ____________________________________
________________________________________________________________________��
(5)д��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ_____________________��
�ٷ����к����Ȼ�
��1HNMR����ʾ�����к��б������ұ����������ֲ�ͬ��ѧ��������ԭ��
(6)ͨ��������ϩΪԭ���Ƶû����������X��Ӧ�ϳ�D�����û�ѧ��Ӧ����ʽ��ʾ����ϩΪԭ���Ʊ�X�ĺϳ�·��(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ʯ�ͼ۸����ǣ���úΪԭ���Ʊ�һЩ������Ʒ��ǰ���ֱ����á���ͼ������AΪԭ������������ë�ĺϳ�·�ߡ�
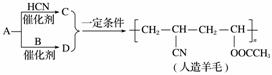
����˵����ȷ���� (����)
A���ϳ�������ë�ķ�Ӧ�������۷�Ӧ
B��A����C�ķ�Ӧ���ڼӳɷ�Ӧ
C��A����D�ķ�Ӧ����ȡ����Ӧ
D����A�Ľṹ��ʽΪCH2===CH2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ŵIJ�ͬ�����Զ��л�����з��࣬��ָ�������л�������࣬���ں����ϡ�
(1)CH3CH2CH2OH________��
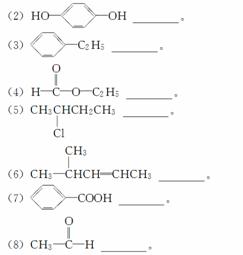
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʲ���Ϊͬ���칹�����(����)
A��2,2���������� 2������
B�����ȼױ��Ͷ��ȼױ�
C��2�����������
D������ϩ��ͼ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵķ����У�������ϵ�����ϡ�X����Y��Y����Z������(����)
| ѡ�� | X | Y | Z |
| A | �����廯���� | �������������� |
|
| B | ֬���廯���� | ��״���������� | CH3COOH(����) |
| C | ��״������ | �����廯���� | ����ͬϵ�� |
| D | �������� | ������ |
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�����������ʵ���˵���������(����)
A����ȥ���������к��е����ᣬ��õĴ�������������������̼������Һϴ�Ӻ��Һ
B������������ʱ�����Ҵ��л�������Ũ���������
C���ɽ�����ͨ�뱥��̼������Һ���ռ���Ӧ���ɵ���������
D��1 mol�Ҵ���2 mol������Ũ����������²��ܺϳ� 1 mol ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com