
��1��49gH2SO4�����ʵ����� ���������Ƴ�200mL��Һ��������Һ�����ʵ���Ũ��Ϊ �����к�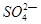 ��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��2��������ء���������������ɵĻ����Һ����PH=1��c(Al3+)=0.4mol/L, c(![]() )=0.8mol/ L, ��c(K+)Ϊ ��
)=0.8mol/ L, ��c(K+)Ϊ ��
��3������״����a L HCl(g)����1000 gˮ�У��õ�������ܶ�Ϊbg/cm3�������������ʵ���Ũ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ�λ���ѧ������ѧ�ڽ�ѧ������⣨һ����ѧ ���ͣ������
��1��49gH2SO4�����ʵ����� ���������Ƴ�200mL��Һ��������Һ�����ʵ���Ũ��Ϊ �����к�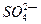 ��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��2��������ء���������������ɵĻ����Һ����PH=1��c(Al3+)="0.4mol/" L, c(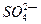 )="0.8" mol/ L, ��c(K+)Ϊ ��
)="0.8" mol/ L, ��c(K+)Ϊ ��
��3������״����a L HCl(g)����1000 gˮ�У��õ�������ܶ�Ϊbg/ cm3�������������ʵ���Ũ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ������ѧ�ڽ�ѧ������⣨һ����ѧ ���ͣ������
��1��49gH2SO4�����ʵ����� ���������Ƴ�200mL��Һ��������Һ�����ʵ���Ũ��Ϊ
�����к�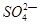 ��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��2��������ء���������������ɵĻ����Һ����PH=1��c(Al3+)=0.4mol/
L, c(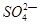 )=0.8
mol/ L, ��c(K+)Ϊ ��
)=0.8
mol/ L, ��c(K+)Ϊ ��
��3������״����a L HCl(g)����1000 gˮ�У��õ�������ܶ�Ϊbg/ cm3�������������ʵ���Ũ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���1��49gH2SO4�����ʵ����� mol ��200ml1.5mol/L��Na2CO3��Һ���������ʵ������� g ��
![]()
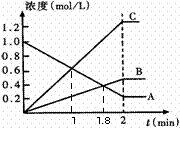 ��2����ͼ��ʾ800��ʱ��A��B��C������������
��2����ͼ��ʾ800��ʱ��A��B��C������������
![]() ��Ũ����ʱ��仯�������t��ʾʱ�䡣
��Ũ����ʱ��仯�������t��ʾʱ�䡣
![]() �Իش�
�Իش�
![]() �ٴﵽƽ��״̬������ʱ���� min��
�ٴﵽƽ��״̬������ʱ���� min��
![]() �ڴӷ�Ӧ��ʼ���ﵽƽ���ʱ���ڣ�A���ʵ�ƽ����Ӧ����Ϊ ��
�ڴӷ�Ӧ��ʼ���ﵽƽ���ʱ���ڣ�A���ʵ�ƽ����Ӧ����Ϊ ��
![]() �� �÷�Ӧ�Ļ�ѧ����ʽΪ ��
�� �÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��49gH2SO4�����ʵ����� ���������Ƴ�200mL��Һ��������Һ�����ʵ���Ũ��Ϊ �����к�![]() ��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��������Һ�����״���µİ��� Lǡ����ȫ��Ӧ����(NH4)2SO4��
��2��������ء���������������ɵĻ����Һ����PH=1��c(Al3+)=0.4mol/ L, c(![]() )=0.8 mol/ L, ��c(K+)Ϊ ��
)=0.8 mol/ L, ��c(K+)Ϊ ��
��3������״����a L HCl(g)����1000 gˮ�У��õ�������ܶ�Ϊbg/ cm3�������������ʵ���Ũ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com