
“ĪĮ×Ėį(H3PO2)ŹĒŅ»ÖÖ¾«ĻøĮ׻ƹ¤²śĘ·£¬¾ßÓŠ½ĻĒ滹ŌŠŌ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)H3PO2ŹĒŅ»ÖÖÖŠĒæĖį£¬Š“³öĘäµēĄė·½³ĢŹ½£ŗ_____________________________
________________________________________________________________________ӣ
(2)H3PO2¼°NaH2PO2¾łæɽ«ČÜŅŗÖŠµÄAg£«»¹ŌĪŖŅų£¬“Ó¶ųæÉÓĆÓŚ»Æѧ¶ĘŅų”£
¢ŁH3PO2ÖŠ£¬PŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ________”£
¢ŚĄūÓĆH3PO2½ųŠŠ»Æѧ¶ĘŅų·“Ó¦ÖŠ£¬Ńõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ4”Ć1£¬ŌņŃõ»Æ²śĪļĪŖ________(Ģī»ÆѧŹ½)”£
¢ŪNaH2PO2ĪŖ________(Ģī”°ÕżŃĪ”±»ņ”°ĖįŹ½ŃĪ”±)£¬ĘäČÜŅŗĻŌ________(Ģī”°ČõĖįŠŌ”±”°ÖŠŠŌ”±»ņ”°Čõ¼īŠŌ”±)”£
(3)H3PO2µÄ¹¤ŅµÖĘ·ØŹĒ£ŗ½«°×Į×(P4)ÓėBa(OH)2ČÜŅŗ·“Ӧɜ³ÉPH3ĘųĢåŗĶBa(H2PO2)2£¬ŗóÕßŌŁÓėH2SO4·“Ó¦”£Š“³ö°×Į×ÓėBa(OH)2ČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ____________________________________________”£
(4)H3PO2Ņ²æÉÓƵēÉųĪö·ØÖʱø£¬”°ĖÄŹŅµēÉųĪö·Ø”±¹¤×÷ŌĄķČēĶ¼ĖłŹ¾(ŃōĤŗĶŅõĤ·Ö±šÖ»ŌŹŠķŃōĄė×Ó”¢ŅõĄė×ÓĶعż)£ŗ
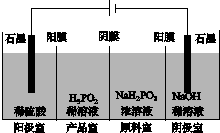
¢ŁŠ“³öŃō¼«µÄµē¼«·“Ó¦Ź½£ŗ_________________________________________”£
¢Ś·ÖĪö²śĘ·ŹŅæɵƵ½H3PO2µÄŌŅņ£ŗ_____________________________________
________________________________________________________________________ӣ
¢ŪŌēĘŚ²ÉÓĆ”°ČżŹŅµēÉųĪö·Ø”±ÖʱøH3PO2£ŗ½«”°ĖÄŹŅµēÉųĪö·Ø”±ÖŠŃō¼«ŹŅµÄĻ”ĮņĖįÓĆH3PO2Ļ”ČÜŅŗ“śĢę£¬²¢³·Č„Ńō¼«ŹŅÓė²śĘ·ŹŅÖ®¼äµÄŃōĤ£¬“Ó¶ųŗĻ²¢ĮĖŃō¼«ŹŅÓė²śĘ·ŹŅ”£ĘäȱµćŹĒ²śĘ·ÖŠ»ģÓŠ________ŌÓÖŹ£¬øĆŌÓÖŹ²śÉśµÄŌŅņŹĒ________________________________”£
(1)H3PO2 H2PO
H2PO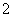 £«H£«””(2)¢Ł£«1””¢ŚH3PO4””¢ŪÕżŃĪ””Čõ¼īŠŌ
£«H£«””(2)¢Ł£«1””¢ŚH3PO4””¢ŪÕżŃĪ””Čõ¼īŠŌ
(3)2P4£«3Ba(OH)2£«6H2O£½3Ba(H2PO2)2£«2PH3”ü
(4)¢Ł2H2O£4e£===O2”ü£«4H£«
¢ŚŃō¼«ŹŅµÄH£«“©¹żŃōĤĄ©É¢ÖĮ²śĘ·ŹŅ£¬ŌĮĻŹŅµÄH2PO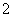 “©¹żŅõĤĄ©É¢ÖĮ²śĘ·ŹŅ£¬¶žÕß·“Ӧɜ³ÉH3PO2””¢ŪPO
“©¹żŅõĤĄ©É¢ÖĮ²śĘ·ŹŅ£¬¶žÕß·“Ӧɜ³ÉH3PO2””¢ŪPO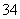 ””H2PO
””H2PO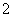 »ņH3PO2±»Ńõ»Æ
»ņH3PO2±»Ńõ»Æ
[½āĪö] (1)H3PO2ĪŖŅ»ŌŖČõĖį£¬ĘäµēĄė·½³ĢŹ½ĪŖH3PO2 H£«£«H2PO
H£«£«H2PO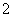 ”£(2)ÓÉ»ÆŗĻ¼Ū“śŹżŗĶĪŖ0æÉČ·¶ØPĪŖ£«1¼Ū£»¢Śøł¾ŻĢāÖŠŠÅĻ¢Š“³ö»Æѧ·½³ĢŹ½ĪŖ4Ag£«£«H3PO2£«2H2O===4Ag£«H3PO4£«4H£«£¬¼“Ńõ»Æ²śĪļĪŖH3PO4£»¢ŪNaH2PO2ĪŖĒæ¼īČõĖįŃĪ£¬ČÜŅŗ³ŹČõ¼īŠŌ”£(3)øł¾ŻĢāÖŠŠÅĻ¢ŗĶ·“Ó¦Ē°ŗóŌŖĖŲ»ÆŗĻ¼Ū±ä»ÆŠ“³ö»Æѧ·½³ĢŹ½ĪŖ2P4£«3Ba(OH)2£«6H2O===2PH3”ü£«3Ba(H2PO2)2”£(4)¢ŁŃō¼«ŹĒĖ®µēĄė³öµÄOH£·Åµē£¬Ęä·“Ó¦Ź½ĪŖ2H2O£4e£===O2”ü£«4H£«£»¢ŚŃō¼«ŹŅÖŠµÄH£«“©¹żŃōĤ½ųČė²śĘ·ŹŅ£¬ŌĮĻŹŅµÄH2PO
”£(2)ÓÉ»ÆŗĻ¼Ū“śŹżŗĶĪŖ0æÉČ·¶ØPĪŖ£«1¼Ū£»¢Śøł¾ŻĢāÖŠŠÅĻ¢Š“³ö»Æѧ·½³ĢŹ½ĪŖ4Ag£«£«H3PO2£«2H2O===4Ag£«H3PO4£«4H£«£¬¼“Ńõ»Æ²śĪļĪŖH3PO4£»¢ŪNaH2PO2ĪŖĒæ¼īČõĖįŃĪ£¬ČÜŅŗ³ŹČõ¼īŠŌ”£(3)øł¾ŻĢāÖŠŠÅĻ¢ŗĶ·“Ó¦Ē°ŗóŌŖĖŲ»ÆŗĻ¼Ū±ä»ÆŠ“³ö»Æѧ·½³ĢŹ½ĪŖ2P4£«3Ba(OH)2£«6H2O===2PH3”ü£«3Ba(H2PO2)2”£(4)¢ŁŃō¼«ŹĒĖ®µēĄė³öµÄOH£·Åµē£¬Ęä·“Ó¦Ź½ĪŖ2H2O£4e£===O2”ü£«4H£«£»¢ŚŃō¼«ŹŅÖŠµÄH£«“©¹żŃōĤ½ųČė²śĘ·ŹŅ£¬ŌĮĻŹŅµÄH2PO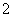 “©¹żŅõĤĄ©É¢ÖĮ²śĘ·ŹŅ£¬¶žÕß·“Ӧɜ³ÉH3PO2£»¢ŪŃō¼«ŹŅÄŚæÉÄÜÓŠ²æ·ÖH2PO
“©¹żŅõĤĄ©É¢ÖĮ²śĘ·ŹŅ£¬¶žÕß·“Ӧɜ³ÉH3PO2£»¢ŪŃō¼«ŹŅÄŚæÉÄÜÓŠ²æ·ÖH2PO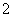 »ņH3PO2Ź§µē×Ó·¢ÉśŃõ»Æ·“Ó¦£¬µ¼ÖĀÉś³ÉĪļÖŠ»ģÓŠPO
»ņH3PO2Ź§µē×Ó·¢ÉśŃõ»Æ·“Ó¦£¬µ¼ÖĀÉś³ÉĪļÖŠ»ģÓŠPO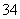 ”£
ӣ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«15 mL 2 mol/L Na2CO3ČÜŅŗÖšµĪ¼ÓČėµ½40 mL 0.5 mol/L MClnŃĪČÜŅŗÖŠ£¬Ē”ŗĆ½«ČÜŅŗÖŠµÄMn£«Ąė×ÓĶźČ«³ĮµķĪŖĢ¼ĖįŃĪ£¬ ŌņMClnÖŠnÖµŹĒ(””””)
ŌņMClnÖŠnÖµŹĒ(””””)
A£®4 B£®3
C£®2 D£®1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«±„ŗĶFeCl3ČÜŅŗµĪČė·ŠĖ®²¢Öó·ŠŅ»¶ĪŹ±¼ä£¬æɵƵ½ŗģŗÖÉ«ŅŗĢ壬“ĖŅŗĢå²»¾ßÓŠµÄŠŌÖŹŹĒ(””””)
A£®¹āŹųĶعżøĆŅŗĢåŹ±ŠĪ³É¹āĮĮµÄ”°ĶØĀ·”±
B£®²åČėŹÆÄ«µē¼«ĶØÖ±Į÷µēŗó£¬ÓŠŅ»¼«ø½½üŅŗĢåŃÕÉ«¼ÓÉī
C£®ĻņøĆŅŗĢåÖŠ¼ÓČėĻõĖįŅųČÜŅŗ£¬ĪŽ³Įµķ²śÉś
D£®½«øĆŅŗĢå¼ÓČČ”¢ÕōøÉ”¢×ĘÉÕŗó£¬ÓŠŃõ»ÆĪļÉś³É
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ė®ČÜŅŗÖŠÄÜ“óĮæ¹²“ęµÄŅ»×éĄė×ÓŹĒ(””””)
A£®Na£«”¢Ca2£«”¢Cl£”¢SO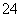
B£®Fe2£«”¢H£«”¢SO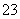 ”¢ClO£
”¢ClO£
C£®K£«”¢Fe3£«”¢NO ”¢SCN£
”¢SCN£
D£®Mg2£«”¢NH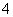 ”¢Cl£”¢SO
”¢Cl£”¢SO
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Įņ»ÆĒāµÄ×Ŗ»ÆŹĒ׏Ō“ĄūÓĆŗĶ»·¾³±£»¤µÄÖŲŅŖŃŠ¾ææĪĢā”£ÓÉĮņ»ÆĒā»ńµĆĮņµ„ÖŹÓŠ¶ąÖÖ·½·Ø”£
(1)½«ÉÕ¼īĪüŹÕH2SŗóµÄČÜŅŗ¼ÓČėµ½ČēĶ¼ĖłŹ¾µÄµē½ā³ŲµÄŃō¼«Ēų½ųŠŠµē½ā”£µē½ā¹ż³ĢÖŠŃō¼«Ēų·¢ÉśČēĻĀ·“Ó¦£ŗ
S2££2e£===S””(n£1)S£«S2£===S

¢ŁŠ“³öµē½āŹ±Ņõ¼«µÄµē¼«·“Ó¦Ź½£ŗ________________”£
¢Śµē½āŗóŃō¼«ĒųµÄČÜŅŗÓĆĻ”ĮņĖįĖį»ÆµĆµ½Įņµ„ÖŹ£¬ĘäĄė×Ó·½³ĢŹ½æÉŠ“³É__________________________”£
(2)½«H2SŗĶæÕĘųµÄ»ģŗĻĘųĢåĶØČėFeCl3”¢FeCl2”¢CuCl2µÄ»ģŗĻČÜŅŗÖŠ·“Ó¦»ŲŹÕS£¬ĘäĪļÖŹ×Ŗ»ÆČēĶ¼ĖłŹ¾”£
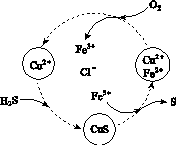
¢ŁŌŚĶ¼Ź¾µÄ×Ŗ»ÆÖŠ£¬»ÆŗĻ¼Ū²»±äµÄŌŖĖŲŹĒ________”£
¢Ś·“Ó¦ÖŠµ±ÓŠ1 mol H2S×Ŗ»ÆĪŖĮņµ„ÖŹŹ±£¬±£³ÖČÜŅŗÖŠFe3£«µÄĪļÖŹµÄĮæ²»±ä£¬ŠčĻūŗÄO2µÄĪļÖŹµÄĮæĪŖ________”£
¢ŪŌŚĪĀ¶ČŅ»¶ØŗĶ²»²¹¼ÓČÜŅŗµÄĢõ¼žĻĀ£¬»ŗĀżĶØČė»ģŗĻĘųĢ壬²¢³ä·Ö½Į°č”£ÓūŹ¹Éś³ÉµÄĮņµ„ÖŹÖŠ²»ŗ¬CuS£¬æɲÉČ”µÄ“ėŹ©ÓŠ________________”£
(3)H2SŌŚøßĪĀĻĀ·Ö½āÉś³ÉĮņÕōĘųŗĶH2”£Čō·“Ó¦ŌŚ²»Ķ¬ĪĀ¶ČĻĀ“ļµ½Ę½ŗāŹ±£¬»ģŗĻĘųĢåÖŠø÷×é·ÖµÄĢå»ż·ÖŹżČēĶ¼ĖłŹ¾£¬H2SŌŚøßĪĀĻĀ·Ö½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________________________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
H2O2ŹĒŅ»ÖÖĀĢÉ«Ńõ»Æ»¹ŌŹŌ¼Į£¬ŌŚ»Æѧъ¾æÖŠÓ¦ÓĆ¹ć·ŗ”£
(1)ijŠ”×éÄāŌŚĶ¬ÅضČFe3£«µÄ“ß»ÆĻĀ£¬Ģ½¾æH2O2ÅØ¶Č¶ŌH2O2·Ö½ā·“Ó¦ĖŁĀŹµÄÓ°Ļģ”£ĻŽŃ”ŹŌ¼ĮÓėŅĒĘ÷£ŗ30%H2O2ČÜŅŗ”¢0.1 mol”¤L£1Fe2(SO4)3ČÜŅŗ”¢ÕōĮóĖ®”¢×¶ŠĪĘ攢Ė«æ×Čū”¢Ė®²Ū”¢½ŗ¹Ü”¢²£Į§µ¼¹Ü”¢ĮæĶ²”¢Ćė±ķ”¢ŗćĪĀĖ®Ō”²Ū”¢×¢ÉäĘ÷”£
¢ŁŠ“³ö±¾ŹµŃéH2O2·Ö½ā·“Ó¦·½³ĢŹ½²¢±źĆ÷µē×Ó×ŖŅʵķ½ĻņŗĶŹżÄæ£ŗ______________________________”£
¢ŚÉč¼ĘŹµŃé·½°ø£ŗŌŚ²»Ķ¬H2O2ÅضČĻĀ£¬²ā¶Ø________(ŅŖĒóĖł²āµĆµÄŹż¾ŻÄÜÖ±½ÓĢåĻÖ·“Ó¦ĖŁĀŹ“óŠ”)”£
¢ŪÉč¼ĘŹµŃé×°ÖĆ£¬Ķź³ÉĶ¼ÖŠµÄ×°ÖĆŹ¾ŅāĶ¼”£
¢Ü²ĪÕÕĻĀ±ķøńŹ½£¬Äā¶ØŹµŃé±ķøń£¬ĶźÕūĢåĻÖŹµŃé·½°ø(ĮŠ³öĖłŃ”ŹŌ¼ĮĢå»ż”¢Šč¼ĒĀ¼µÄ“ż²āĪļĄķĮæŗĶĖłÄā¶ØµÄŹż¾Ż£»Źż¾ŻÓĆ×ÖÄø±ķŹ¾)”£
| ””””ĪļĄķĮæ ŹµŃéŠņŗÅ ”””” | V[0.1 mol”¤L£1 Fe2(SO4)3]/mL | ”” | |
| 1 | a | ”” | |
| 2 | a | ”” |
(2)ĄūÓĆĶ¼(a)ŗĶ(b)ÖŠµÄŠÅĻ¢£¬°“Ķ¼(c)×°ÖĆ(Į¬ĶصÄA”¢BĘæÖŠŅŃ³äÓŠNO2ĘųĢå)½ųŠŠŹµŃ锣æɹŪ²ģµ½BĘæÖŠĘųĢåŃÕÉ«±ČAĘæÖŠµÄ__________(Ģī”°Éī”±»ņ”°Ē³”±)£¬ĘäŌŅņŹĒ____________________________”£
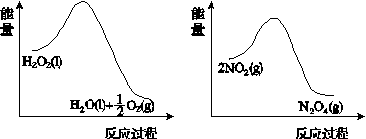
””””””””””(a)””””””””””””””””””””””””(b)
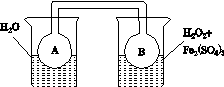
(c)
Ķ¼21
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¼īŹ½Ģ¼ĖįĀĮĆ¾
[MgaAlb(OH)c(CO3)d”¤xH2O]³£ÓĆ×÷ĖÜĮĻ×čČ¼¼Į”£
(1)¼īŹ½Ģ¼ĖįĀĮĆ¾¾ßÓŠ×čČ¼×÷ÓĆ£¬ŹĒÓÉÓŚĘäŹÜČČ·Ö½āŠčĪüŹÕ“óĮæČČĮæŗĶ________________________________”£
(2)MgaAlb(OH)c(CO3)d”¤xH2OÖŠa”¢b”¢c”¢dµÄ“śŹż¹ŲĻµŹ½ĪŖ________”£
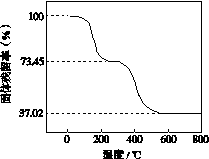
(3)ĪŖČ·¶Ø¼īŹ½Ģ¼ĖįĀĮĆ¾µÄ×é³É£¬½ųŠŠČēĻĀŹµŃé£ŗ
¢Ł×¼Č·³ĘČ”3.390 g ѳʷÓė×ćĮæĻ”ŃĪĖį³ä·Ö·“Ó¦£¬Éś³ÉCO2 0.560 L(ŅŃ»»Ėć³É±ź×¼×“æöĻĀ)”£
¢ŚĮķČ”Ņ»¶ØĮæѳʷŌŚæÕĘųÖŠ¼ÓČČ£¬ŃłĘ·µÄ¹ĢĢ岊ĮōĀŹ(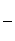 ”Į100%)ĖęĪĀ¶ČµÄ±ä»ÆČēĶ¼ĖłŹ¾(ѳʷŌŚ270 ”ꏱŅŃĶźČ«Ź§Č„½į¾§Ė®£¬600 ”ęŅŌÉĻ²ŠĮō¹ĢĢåĪŖ½šŹōŃõ»ÆĪļµÄ»ģŗĻĪļ)”£
”Į100%)ĖęĪĀ¶ČµÄ±ä»ÆČēĶ¼ĖłŹ¾(ѳʷŌŚ270 ”ꏱŅŃĶźČ«Ź§Č„½į¾§Ė®£¬600 ”ęŅŌÉĻ²ŠĮō¹ĢĢåĪŖ½šŹōŃõ»ÆĪļµÄ»ģŗĻĪļ)”£
øł¾ŻŅŌÉĻŹµŃ鏿¾Ż¼ĘĖć¼īŹ½Ģ¼ĖįĀĮĆ¾ŃłĘ·ÖŠµÄn(OH£)”Ćn(CO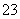 )(Š“³ö¼ĘĖć¹ż³Ģ)”£
)(Š“³ö¼ĘĖć¹ż³Ģ)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
±Č½ĻŅŅĶéŗĶŅŅ“¼µÄ½į¹¹£¬ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ(””””)
A£®Į½øöĢ¼Ō×ÓŅŌµ„¼üĻąĮ¬
B£®·Ö×ÓĄļ¶¼ŗ¬ÓŠ6øöĻąĶ¬µÄĒāŌ×Ó
C£®ŅŅ»łÓėŅ»øöĒāŌ×ÓĻąĮ¬¾ĶŹĒŅŅĶé·Ö×Ó
D£®ŅŅ»łÓėŅ»øöōĒ»łĻąĮ¬¾ĶŹĒŅŅ“¼·Ö×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØÄÜŌŚĻĀĮŠČÜŅŗÖŠ“óĮæ¹²“ęµÄĄė×Ó×éŹĒ(””””)
A£®ŗ¬ÓŠ“óĮæAl3£«µÄČÜŅŗ£ŗNa£«”¢NH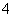 ”¢SO
”¢SO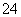 ”¢Cl£
”¢Cl£
B£®c(H£«)£½1”Į10£13 mol/LµÄČÜŅŗ£ŗNa£«”¢Ca2£«”¢SO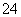 ”¢CO
ӢCO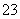
C£®ŗ¬ÓŠ“óĮæFe3£«µÄČÜŅŗ£ŗNa£«”¢Mg2£«”¢NO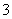 ”¢SCN£
”¢SCN£
D£®ŗ¬ÓŠ“óĮæNO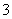 µÄČÜŅŗ£ŗH£«”¢Fe2£«”¢SO
µÄČÜŅŗ£ŗH£«”¢Fe2£«”¢SO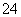 ”¢Cl£
”¢Cl£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com