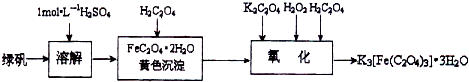
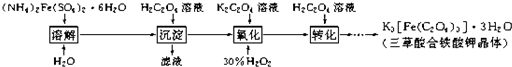
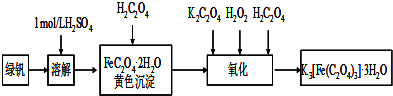
���������������ؾ��壨K
3[Fe��C
2O
4��
3]?3H
2O���к���Ҫ����;��������ͼ�������Ʊ�����������������и��⣺
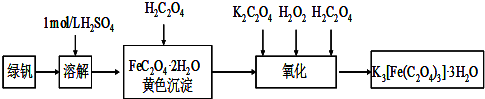
��1����������ϡ�����Ʊ��̷���FeSO
4?7H
2O������������
��
��
�����������ƣ�����Ҫ������������
��ֹFe2+������
��ֹFe2+������
��
��2��Ҫ����Һ�еõ��̷���������е�ʵ�������
bcae
bcae
������ǰ��˳���
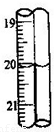
a������ϴ�� b������Ũ�� c����ȴ�ᾧ d������ e������
ij��ȤС��Ϊ�ⶨ�����������ؾ��壨K
3[Fe��C
2O
4��
3]?3H
2O������Ԫ�غ�������������ʵ�飺
����1������5.000g�����������ؾ��壬���Ƴ�250ml��Һ��
����2��ȡ������Һ25.00ml����ƿ�У���ϡH
2SO
4�ữ���μ�KMnO
4��Һ�������ǡ��ȫ���������ɶ�����̼��ͬʱ��MnO
4-����ԭ��Mn
2+����Ӧ�����Һ�м���һ����п�ۣ���������ɫ�պ���ʧ�����ˣ�ϴ�ӣ������˼�ϴ��������Һ�ռ�����ƿ�У���ʱ����Һ�������ԣ�
����3�������������£���0.010mol/L KMnO
4��Һ�ζ������������Һ���յ㣬��������ʵ�飬ƽ������KMnO
4��Һ20.00ml���ζ���MnO
4-������ԭ��Mn
2+��
��3������1�У�������������������Һ��Ҫʹ�õIJ����������ձ��������������
250ml����ƿ
250ml����ƿ
����Ҫ�������������ǣ��������ܽ⡢ת�ơ�
ϴ��
ϴ��
�����ݡ�ҡ�ȣ�
��4������2�У�����п�۵�Ŀ����
��ԭFe3+ΪFe2+
��ԭFe3+ΪFe2+
��
��5������3�У�������Ӧ�����ӷ���ʽΪ��
MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O
MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O
��
��6������2���������KMnO
4����Һ�������������õ�������
ƫ��
ƫ��
����ѡ�ƫ�͡�����ƫ�ߡ��������䡱��
��7��ijͬѧ��8.74g��ˮ�����������أ�K
3[Fe��C
2O
4��
3]����һ�������¼��ȷֽ⣬���ù��������Ϊ5.42g��ͬʱ�õ��ܶ�Ϊ1.647g/L�����ۺϳɱ�״���£����壮�о���������֪����Ԫ�ز�������������ʽ���ڣ�����ֻ��K
2CO
3��д���÷ֽⷴӦ�Ļ�ѧ����ʽ
2K3[Fe��C2O4��3]�T3K2CO3+Fe+FeO+4CO+5CO2
2K3[Fe��C2O4��3]�T3K2CO3+Fe+FeO+4CO+5CO2
��