
��ͼ�Dz��ֳ���Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵIJ��ֲ�������ȥ������֪��EΪ����ɫ���壬KΪdz��ɫ��Һ����Ӧ���ǻ��������е���Ҫ��Ӧ��B��C��D��H�ǵ��ʣ�B��C��D��F��G��H����������̬�� F��P ��H��ˮ��Һ������Ư�����ã���F���γ��������Ҫ����֮һ��N��һ�ֳ����ĵ��ʣ�������GΪ�������������������M������Ԫ����ɣ������ڹ���58�����ӡ�
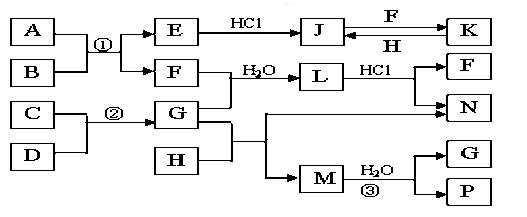
��1��������A�к��е�����Ԫ���� ��дԪ�ط��ţ�M�Ļ�ѧʽ_______
��2����μ��黯����N�е�������
��3��д��K��H��Ӧ�����ӷ���ʽ��
C��D��Ӧ�Ļ�ѧ����ʽ��
��4�������ʵ���F��H�Ļ������ͨ��Ʒ����Һ�е�����Ϊ ��ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ��
��5��ʵ���п���NaOH��Һ�����ն����H����д�������ӷ�Ӧ����ʽ
(2�֣�
��S��Fe(2��)�� NCl3 (1��)
�ƽ�NaOH������뵽N����Һ�в�����,�����������ʹʪ��ĺ�ɫʯ����ֽ����.
NH3��H2O = NH3��+H2O (2��) (����������Ҳ����)
| ||||||||||||||||||||||