



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
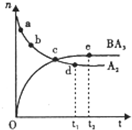 A��B��C��D��E��F����Ԫ�ط������������ڣ���ԭ��������������A��ԭ�������ڱ��а� ����С��A��D�����壬���γ����ӻ�����DA��C��Fͬ���壬���γ�FC2��FC3���ַ���B��D��E ���ߵ�����������Ӧ��ˮ��������֮����ɷ�Ӧ���ɿ������κ�ˮ���������о���CԪ �أ�����д���пհף�
A��B��C��D��E��F����Ԫ�ط������������ڣ���ԭ��������������A��ԭ�������ڱ��а� ����С��A��D�����壬���γ����ӻ�����DA��C��Fͬ���壬���γ�FC2��FC3���ַ���B��D��E ���ߵ�����������Ӧ��ˮ��������֮����ɷ�Ӧ���ɿ������κ�ˮ���������о���CԪ �أ�����д���пհף��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�Ž�һ�и�����һ���¿���ѧ�Ծ� ���ͣ������
��6�֣���һ�ݻ�Ϊ2L���ݻ�������ܱ������м�������̼�ۺ�0.2molH2O����800�������£���20s��ﵽ���»�ѧƽ�⣺C(s) +H2O(g)  CO(g) + H2(g)����H=" -Q" kJ��mol-1��Q>0������֪�ﵽƽ��ʱ��COΪ0.12mol���Իش�
CO(g) + H2(g)����H=" -Q" kJ��mol-1��Q>0������֪�ﵽƽ��ʱ��COΪ0.12mol���Իش�
��1�������������䣬����������̼����ƽ���ƶ�����ǣ�________________��
��2�����æ�(H2O)��ʾ�÷�Ӧǰ20s�ڵ�ƽ�����ʣ����(H2O)=" ________________ " ��
��3����������ƽ��Ļ�������ٳ���0.08mol H2������ͬ�����´ﵽ�µ�ƽ�⣬��ʱCO�����ʵ���Ϊ________________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ��ʦ��һ���и߿���ѧģ���Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com