
4,7-�����㶹�أ��۵㣺132.6�棩��һ����Ҫ�����ϣ��㷺�ֲ���ֲ�����,�ɼ�ױ���Ϊԭ�ϵĺϳɷ�Ӧ���£�
ʵ��װ��ͼ���£�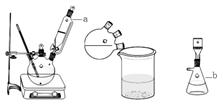
��Ҫʵ�鲽�裺
����1.��������ƿ�м���60mLŨ���ᣬ����ȴ��0�����£������µ����ױ���30mL(0.29mol)��������������26.4mL (0.21mol)�Ļ���
����2.������10���£�����12h����Ӧ��ȫ���䵹���ˮ������У�Ȼ����ˡ�ˮϴ�ô�Ʒ
����3.��Ʒ���Ҵ��ܽⲢ�ؽᾧ���ð�ɫ��״���岢��ɣ��Ƶò�Ʒ����Ϊ33.0g��
��1��ͼ����Ʒ���ƣ�a ��b ��
��2��ŨH2SO4��Ҫ��ȴ��0�����µ�ԭ���� ��
��3����Ӧ��Ҫ����12h����ԭ���� ��
��4��ȷ�����ղ�Ʒ��4,7-�����㶹�ص�ʵ����� ��
��5������ʵ�����Ϊ ��
��1����ѹ��Һ©����2�֣�������ƿ��2�֣���
��2����ֹŨ���Ὣ�л���������̿����2�֣���
��3��ʹ��Ӧ���ֽӴ���Ӧ����߷�Ӧ���ʣ�2�֣�/
��4�������۵��ⶨ����⣨���⣩���ף����˴Ź������ף��ȣ�2�֣���
��5��89.0%��2�֣���
���������������1����ѹ��Һ©��������ƿ����2��Ũ�������ǿ�����Ժ������ԣ��¶ȸ���ʹ�л�����������������������ˮ̼������3��ʹ��Ӧ���ֽӴ���Ӧ����߷�Ӧ���ʣ���4��4,7-�����㶹�أ��۵㣺132.6�棩�۵�ϵͣ����Բ����۵㣬Ҳ����ͨ��ʵ�������ⶨ����⣨���⣩���ף����˴Ź������ף��ȣ���5�����۲���Ϊ0.21��176=36.96g������Ϊ33.0��36.96=0.89
���㣺����ʵ�黯ѧ�������Ʊ�ԭ�����ʵ��й����⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ӷϷ���������Ҫ�ɷ�V2O5��VOSO4��K2SO4��SiO2�ȣ��л���V2O5��һ��������������ʾ��ͼ���£���ش��������⣺
��1��������з�������Ҫ�ɷ��� ������X�Լ�Ϊ ��
��2��ʵ�����н�����ȡ��Һ����ʱ��ע����ȡ�������������Һ©������Ȧ�Ͼ��ã���Һ��ֲ�������IJ����� ��
��3���ڡ��۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������
R2(SO4)n (ˮ��)+ 2nHA���л��㣩 2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��
2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��
��4������ɢ��еķ�Ӧ���ӷ���ʽ��
��ClO3- + ��VO2+ +��H+ =��VO3+ + �� +��
��5��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�ij������;�㷺�������������Լ���ýȾ��������������ԭ�ϡ����ⶨ��������Ԫ�أ�Ħ������Ϊ482g/mol��Ϊ��һ��ȷ��������ɣ�ij��ѧ��ȤС����������ʵ�飺
��ȡ48.20g����������ˮ�����100mL��Һ��������Һ���ػ�ɫ��
��ȡ������Һ50mL���Թ��У�����������0.1mol/LNaOH��Һ�������ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g�������ĺ��ɫ�����������ˡ�ϴ�ӡ����պ��4.00g���塣
����ȡ������Һ50mL���Թ��У�����������BaCl2��Һ����������������İ�ɫ���� 23.30g��
��ش��������⣺
��1��ʵ����в�������ĵ���ʽ ��
��2�������ʵĻ�ѧʽΪ �������йظ����ʵ���;�������� ��
| A����Ѫ�� | B����ˮ�� | C�����ӷ�ˮ�ļ���Լ� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��16�֣������Ѱ�ҵ�ĸ���ƷFeSO4����TiO2+��Al3+������������ؼ��ߴ���ϸ�����������乤���������£�
��1������FeSO4�Ƿ����в��������ķ����� ��
��2����֪����1�õ�����������Ҫ�ɷ���Al(OH)3��H2TiO3���벹�仯ѧ����ʽ��
TiOSO4 + ��H2SO4 + H2TiO3�������۵������У��ٳ�ȥ��Һ�е�Fe3+���� ��
��3��������Ӧ�����ӷ���ʽ�� ��
��4���������̵ķ�Ӧ�¶�Ϊ40�棬�¶Ȳ��˹��ߵ�ԭ����˿��Ƴ����������⣬���� ��FeC2O4���ɺ�Ϊ��߲�Ʒ���ȣ����������ҺpH��2����pH���ͣ�����FeC2O4�IJ���______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��5������2�õ�����Һ������Ũ���� ��ϴ�ӿɵõ�����Ʒ�������ʿ����� (д��һ����;)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣�����ѧ������ѧ�뼼����
�ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO��Cr2O3������ΪSiO2��Al2O3��Ϊԭ��������ʵ����ģ�ҵ���ø�������K2Cr2O7����Ҫ��������ͼ���漰����Ҫ��Ӧ��: 6FeO��Cr2O3+24NaOH+7KClO3=12Na2CrO4+3Fe2O3+7KCl+12H2O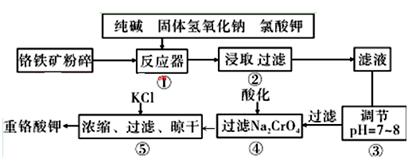
��1�����ǰ������������Ŀ���� ��
��2������۵���pH����˵õ��������� ��
��3���������У��ữʱ��CrO42-ת��ΪCr2O72-��д��ƽ��ת�������ӷ���ʽ ��
��4���ü�Ҫ������˵�������ݼ���KC1��ԭ��
��5��Ŀǰ��������Cr2O72-��ˮ������������巨���÷������ˮ�м���FeSO4 ��7H2O��Cr2O72-��ԭ��Cr3+������pH��Fe��Crת�����൱�� ����������,�������ֱ�ʾԪ�ؼ�̬���ij���������1mol Cr2O72-�������a mol FeSO4 ? 7H2O�����н�����ȷ����_______��
����������,�������ֱ�ʾԪ�ؼ�̬���ij���������1mol Cr2O72-�������a mol FeSO4 ? 7H2O�����н�����ȷ����_______��
| A��x ="0.5" ,a =8 | B��x ="0.5" ,a =" 10" | C��x =" 1.5" ,a =8 | D��x =" 1.5" ,a = 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijС��ͬѧ��һ��Ũ��NaHCO3��Һ���뵽CuSO4��Һ�з��������˳�������ͬѧ��Ϊ������CuCO3����ͬѧ��Ϊ������CuCO3��Cu(OH)2�Ļ����������ʵ��ⶨ������CuCO3������������
(1)���ռ�ͬѧ�Ĺ۵㣬������Ӧ�����ӷ���ʽΪ ��
(2)��ͬѧ��Ϊ��Cu(OH)2���ɵ����������� (�����ӷ���ʽ��ʾ)��
(3)��ͬѧ������ͼ��ʾװ�ý��вⶨ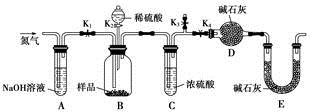
�����о����������ǰ���뽫��������Һ�з��벢�����������������Ϊ ��ϴ�ӡ����
��װ��E�м�ʯ�ҵ������� ��
��ʵ������������²������裺
a���ر�K1��K3����K2��K4����ַ�Ӧ
b����K1��K4���ر�K2��K3��ͨ���������
c����K1��K3���ر�K2��K4��ͨ���������
��ȷ��˳���� (��ѡ����ţ���ͬ)��
��δ���в��� ����ʹ�������ƫ�͡�
����������Ʒ������Ϊm g��װ��D������������n g���������CuCO3����������Ϊ ��
(4)��ͬѧ��Ϊ������ͨ������CO2���������� ���ⶨ������CuCO3������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС����ʵ��������CaSO4��NH3��CO2�Ʊ�(NH4)2SO4���乤���������¡�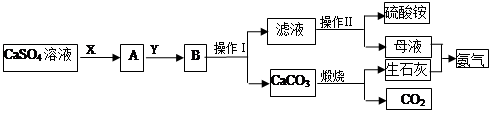
�ش��������⣺
��1�������������Ϊ_________��������һϵ�в�����������Ũ����________�����ˡ�
��2��ʵ����������̼���ʱ��ʢ��̼������õ�������________(������)��
��3��X����Ϊ____(�ѧʽ����ͬ)��Y����Ϊ____����ѭ�����õ����ʵ���_____��
��4��Ҫ�ⶨ���Ƶõ�����林��ȣ�ȡ10.0g��Ʒ����ȫ����ˮ������Һ�еμӹ������Ȼ�����Һ�����ˡ�ϴ�ӡ������������������Ϊ16.31g��Ϊ���������������Ȼ�����Һ�Ƿ������õ��Լ���_______�����Ƶ�����淋Ĵ���Ϊ________��
��5������װ�ò�������ʵ�����ư�������__________(�����)��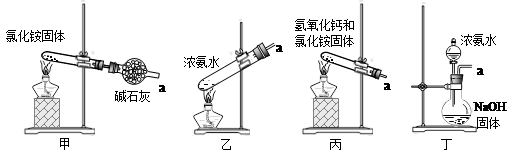
ѡ��������ȡװ�ú���������װ���ռ�����İ���������ȡ�������Һ�����ӵ�˳��(�ýӿ������ĸ��ʾ)�ǣ�a��____��____��____��____��_____��____��_____��
����װ����CCl4��������___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС��Ի�ԭ������ˮ�����ķ�Ӧ������п�ѧ̽������֪Ca(OH)2�ķֽ��¶�Ϊ580�棬������ˮ������Ӧ���¶�Ϊ900�棺������ͼ��ʾʵ��װ�ã������˻�ԭ������ˮ�����ķ�Ӧʵ�飬ʵ���й۲쵽����Һ�в����˴��������ݡ�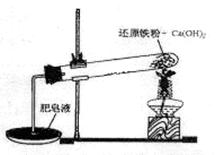
��1��ʵ����Ca(OH)2�������� ��ʵ���в�������������� ��
��2��Ϊ��һ��̽����ԭ������ˮ������Ӧ�������ijɷ֣��о���ѧϰС�齫��Ӧ��Ĺ��徭������õ���ɫ��������壬��Ժ�ɫ��������壬��С��������µļ��貢��������ص�ʵ�飺
����һ������ΪFeO
�����������ΪFe3O4
��������
����ѡ�������Լ������ᡢKSCN��Һ��K3Fe(CN)6 ��Һ����ˮ��֤������һ������
| ���� | ���� | ���� |
| | | ����һ������ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������;�㷺����������ʳƷ���ʼ���ʳƷ���Ӽ����л��ϳ��м���ȡ���һ�ֺϳ�ԭ�����£�
��ʵ�鲽�衿
����A�����μ����ʯ��һ�������ı���ȩ��������������̼��ء�
�ڿ����¶�1500C~1700C��ʹ���ַ�Ӧ��
������ȴ�������ƿ�ڼ��뱥��̼������Һ������pH��9~10��
����װ��B��ʾ����ˮ��������ȥδ��Ӧ�ı���ȩ��
�ݼ������̿������������ɫ��
�ޡ���
��1��װ��A���������� �ˣ��a����b����ͨ������ˮ��
��2��������м��뱥��̼������Һ������ᡢ����ת��Ϊ������ƺʹ����Ƶ�ԭ�� ��
��3��װ��B�ڽ���ˮ��������֮ǰ������еIJ���Ϊ �������ܵ�����Ϊ ��
��4�����۲쵽�������� ��˵��ˮ�������������
��5�������ͨ�����²������롢�ᴿ�ýϴ���������ᣨ������ˮ��������ȷ�IJ���˳���� ������ĸ����
a���ؽᾧ b����ȴ�����ˣ�ˮϴ����
c������Ũ�������pH=3 d�����ã����ȹ��˵����������Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com