
 ��֪ A��B��DΪ��ѧ�����ĵ��ʣ��ס��ҡ�����������Ϊ������Ԫ����ɵĻ�������У�����һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壻����һ�ָ���ȼ�ϣ������Ԫ�������ͬ��1mol�������в�ͬԭ�ӵ���Ŀ��Ϊ1��2���Һ���18mol���ӣ�����һ��������ˮ�İ�ɫ��״���ʣ�������ǿ�ᷴӦ��Ҳ����ǿ�Ӧ�����о�ˮ���ã������ʼ��ת����ϵ����ͼ��ʾ��ijЩ��������ȥ������ش�
��֪ A��B��DΪ��ѧ�����ĵ��ʣ��ס��ҡ�����������Ϊ������Ԫ����ɵĻ�������У�����һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壻����һ�ָ���ȼ�ϣ������Ԫ�������ͬ��1mol�������в�ͬԭ�ӵ���Ŀ��Ϊ1��2���Һ���18mol���ӣ�����һ��������ˮ�İ�ɫ��״���ʣ�������ǿ�ᷴӦ��Ҳ����ǿ�Ӧ�����о�ˮ���ã������ʼ��ת����ϵ����ͼ��ʾ��ijЩ��������ȥ������ش�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧʽ | CH3COOH | HClO | H2CO3 |
| Ka | Ka=1.8��10-5 | Ka=3.0��10-6 | K a1=4.1��10-7 K a2=5.6��10-11 |
| A����ͬŨ����CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ�ǣ�c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� |
| B��̼������Һ�еμ�������ˮ�����ӷ���ʽΪ��CO32-+Cl2+H2O�THCO3-+Cl-+HClO |
| C����0.1mol?L-1 CH3COOH��Һ�еμ�NaOH ��Һ��ǡ����ȫ�кͣ�����Ũ�ȴ�С��ϵ��c��Na+ ����c��CH3COO-����c��OH-����c��H+�� |
| D��CO32-��ˮ�ⳣ����K a1�ij˻�ΪKw |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڢܢ� | B���٢ۢ� |
| C���٢ڢۢ� | D���٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Խǿ�ĺ��������Ƭ��Ӧ��������Խ�� |
| B������ľ�Һ���炙��ʩ�ã���ʹ��Ч���� |
| C��Mg��OH��2��Al��OH��3�����ֽ⣬������������ȼ�� |
| D��ij����ˮ����һ��ʱ�䣬��pH��4.68��Ϊ4.28����Ϊˮ���ܽ��˽϶��CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
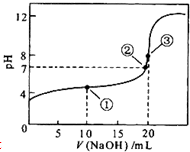 �����£���0.1mol?L-1 NaOH��Һ�ζ�20mL 0.1mol?L-1CH3COOH��Һ�ĵζ�������ͼ��ʾ������˵����ȷ���ǣ�������
�����£���0.1mol?L-1 NaOH��Һ�ζ�20mL 0.1mol?L-1CH3COOH��Һ�ĵζ�������ͼ��ʾ������˵����ȷ���ǣ�������| A�������ʾ��Һ�У�c��Na+����c��CH3COO-����c��CH3COOH����c��H+����c��OH-�� | ||
| B�������ʾ��Һ�У�c��Na+��+c��H+��=c��CH3COO-��+c��CH3COOH��+c��OH-�� | ||
| C�������ʾ��Һ�У�c��CH3COO-����c��Na+����c��OH-����c��H+�� | ||
D���������ζ������У���Һ��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ƽ��ȡ4.9gNaOH���� |
| B��NaOH���������ˮ�ܽ⣬Ҫ����Һ��ȴ�����º���ת��������ƿ�� |
| C������ƿ�����ò���ƿ����Ӧ������ƿ�� |
| D������ҡ�Ⱥ�����Һ������ڿ̶��ߣ��ٲ�����������ˮ���̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������������װ�С���ת��������ʹ�ж���CO��NO��Ӧ����N2��CO2 |
| B����ú�м�������ʯ��ʯ��ʹúȼ�ղ�����SO2��������CaSO3���ɼ��ٶԴ�������Ⱦ |
| C���ߴ��ȵĹ赥�ʹ㷺�����������ά |
| D������ȼ�ջ�ʯȼ���ŷŵķ����к�CO2��SO2���Ӷ�ʹ��ˮ��pH=5.6�γ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com