
����̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӡ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ���⡣
��1��д��CO2��H2��Ӧ����CH4��H2O���Ȼ�ѧ����ʽ ��
��֪�� �� CO(g)+H2O(g) H2(g)+CO2(g) ��H����41kJ��mol��1
H2(g)+CO2(g) ��H����41kJ��mol��1
�� C(s)+2H2(g) CH4(g) ��H����73kJ��mol��1
CH4(g) ��H����73kJ��mol��1
�� 2CO(g) C(s)+CO2(g) ��H����171kJ��mol��1
C(s)+CO2(g) ��H����171kJ��mol��1
��2����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2(g) + 6H2(g) CH3OCH3(g) + 3H2O(g)����֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯����������ͼ��
CH3OCH3(g) + 3H2O(g)����֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯����������ͼ��
����������������ʱ��������ͼ�л���ƽ��ʱCH3OCH3�����������Ͷ�ϱ�[n(H2) / n(CO2)]�仯������ͼ��
��ij�¶��£���2.0molCO2(g)��6.0molH2(g)�����ݻ�Ϊ2L���ܱ������У���Ӧ����ƽ��ʱ���ı�ѹǿ���¶ȣ�ƽ����ϵ��CH3OCH3(g)�����ʵ��������仯�����ͼ��ʾ�������¶Ⱥ�ѹǿ�Ĺ�ϵ�ж���ȷ���� ��
A. P3��P2��T3��T2 B. P1��P3��T1��T3 C. P2��P4��T4��T2 D. P1��P4��T2��T3
���ں����ܱ������ﰴ�����Ϊ1:3���������̼���� ����һ�������·�Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ�����������б仯��˵��ƽ��һ�����淴Ӧ�����ƶ����� ��
A. ����Ӧ������������С
B. �淴Ӧ������������С
C. ��ѧƽ�ⳣ��Kֵ����
D. ��Ӧ�������ٷֺ�������
E. ���������ܶȼ�С
F. ������ת���ʼ�С
��3�������ѧ���ٴ��������ɫ��ѧ�����룺�ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧ��ʹ�����е�CO2ת��Ϊ������ȼ�ϼ״����״�������ȼ�ϵ�أ�д����ϡ����Ϊ����ʼ״�ȼ�ϵ�ظ�����Ӧʽ__ ���Դ�ȼ�ϵ����Ϊ��ӵ�Դ��ͼ��ʾ�������ͭ��Һ�������ʼʱʢ��1000mL pH��5������ͭ��Һ��25�棬CuSO4��������һ��ʱ�����Һ��pH��Ϊ1����ʱ�ɹ۲쵽�������� ����Ҫʹ��Һ�ָ�����ʼŨ�ȣ��¶Ȳ��䣬������Һ����ı仯����������Һ�м��� �����������ƣ���������ԼΪ g��
��1��CO2(g)+4H2(g) CH4(g)+2H2O(g) ��H����162kJ��mol��1 ��2�֣�
CH4(g)+2H2O(g) ��H����162kJ��mol��1 ��2�֣�
(2) �ٻ�ͼ����ͼ����2�֣�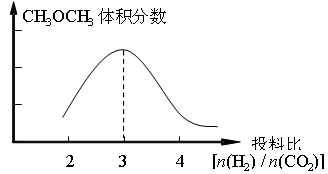
��BD ��2�֣� �� B ��2�֣�
��3��CH3OH+H2O-6e-=CO2��+6H+ ��2�֣�ʯī�缫���������ݲ��������缫�ϸ���һ���ɫ���ʣ���Һ��ɫ��dz��3�֣���3��������֣�������ͭ����̼��ͭ����1�֣���4g����6.2g����1�֣�
���������������1����֪���� CO(g)+H2O(g) H2(g)+CO2(g) ��H����41kJ��mol��1���� C(s)+2H2(g)
H2(g)+CO2(g) ��H����41kJ��mol��1���� C(s)+2H2(g) CH4(g) ��H����73kJ��mol��1���� 2CO(g)
CH4(g) ��H����73kJ��mol��1���� 2CO(g) C(s)+CO2(g) ��H����171kJ��mol��1������ݸ�˹���ɿ�֪���ۣ��١�2+�ڼ��õ�CO2��H2��Ӧ����CH4��H2O���Ȼ�ѧ����ʽO2(g)+4H2(g)
C(s)+CO2(g) ��H����171kJ��mol��1������ݸ�˹���ɿ�֪���ۣ��١�2+�ڼ��õ�CO2��H2��Ӧ����CH4��H2O���Ȼ�ѧ����ʽO2(g)+4H2(g) CH4(g)+2H2O(g) ��H����162kJ��mol��1��
CH4(g)+2H2O(g) ��H����162kJ��mol��1��
��2���ٸ���ͼ���֪CO2��ƽ��ת�������¶�һ������������Ͷ�ϱȵ�����������ݷ���ʽ��֪Ͷ�ϱȣ�3ʱ������ĺ�����ߣ�������ȻCO2��ƽ��ת�������¶�һ������������Ͷ�ϱȵ���������������ѵ��������ֻ����Ͷ�ϱȣ�3ʱ�������ͼ����Ա�ʾΪ���𰸡�
�ڶ��ڷ�Ӧ��2CO2(g) + 6H2(g) CH3OCH3(g) + 3H2O(g)������ѹǿ��ƽ��������Ӧ�����ƶ���������ѵ����ʵ�������Խ�������¶ȶ�����̼��ת���ʽ��ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ���������¶�ƽ�����淴Ӧ�����ƶ��������ѵ����ʵ�������ԽС������P1��P2��P3��P4��T1��T2��T3��T4����ѡBD��
CH3OCH3(g) + 3H2O(g)������ѹǿ��ƽ��������Ӧ�����ƶ���������ѵ����ʵ�������Խ�������¶ȶ�����̼��ת���ʽ��ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ���������¶�ƽ�����淴Ӧ�����ƶ��������ѵ����ʵ�������ԽС������P1��P2��P3��P4��T1��T2��T3��T4����ѡBD��
��A.����Ӧ������������С��˵����Ӧ������Ӧ�����ƶ���A����ȷ��B. �淴Ӧ������������С��˵����Ӧ���淴Ӧ�����ƶ���B��ȷ��C.��ѧƽ�ⳣ��Kֵ����˵��ƽ��������Ӧ�����ƶ���C����ȷ��D. ��Ӧ�������ٷֺ�������˵����Ӧ������Ӧ�����ƶ���D����ȷ��E. �ܶ��ǻ�����������������ݻ��ı�ֵ���ڷ�Ӧ�������������ݻ�ʼ���Dz���ģ���˻��������ܶ�ʼ�ղ��䣬E����ȷ�� F. ������ת���ʼ�С����ƽ�ⲻһ�����淴Ӧ�����ƶ�������ͨ��������������ת����Ҳ�ϵͣ�F����ȷ����ѡB��
��3��ԭ����и���ʧȥ���ӷ���������Ӧ������ϡ����Ϊ����ʼ״�ȼ�ϵ���м״��ڸ���ͨ�룬������ӦʽΪCH3OH+H2O-6e-=CO2��+6H+������װ��ͼ��֪��ʯī����������Һ�е�OH���ŵ�ų�������������������Һ�е�ͭ���ӷŵ�����ͭ������ʵ��������ʯī�缫���������ݲ��������缫�ϸ���һ���ɫ���ʣ���Һ��ɫ��dz����������������ͭ��ϡ���ᣬ������Ҫʹ��Һ�ָ�����ʼŨ�ȣ��¶Ȳ��䣬������Һ����ı仯����������Һ�м�������ͭ��̼��ͭ����Һ�������ӵ����ʵ�����0.1mol/L��1L��0.1mol������ݷ���ʽ2CuSO4��2H2O 2H2SO4��2Cu��O2����֪����Ҫ����ͭ�����ʵ�����0.1mol��2��0.05mol��������0.05mol��80g/mol��4.0g����̼��ͭ����������0.05mol��124/mol��6.2g��
2H2SO4��2Cu��O2����֪����Ҫ����ͭ�����ʵ�����0.1mol��2��0.05mol��������0.05mol��80g/mol��4.0g����̼��ͭ����������0.05mol��124/mol��6.2g��
���㣺�����Ȼ�ѧ����ʽ����д�����������ƽ��״̬���жϡ��绯ѧԭ����Ӧ�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ƻ�1 mol N��N����H��H����N��H���ֱ���Ҫ���յ�����Ϊ946 kJ��436 kJ��391 kJ������1 mol N2(g)��3 mol H2(g)��ȫת��ΪNH3(g)�������仯����ֵΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ķ�������Ϣ���ش����⣺
��1����֪NO2��N2O4�Ľṹʽ�ֱ�Ϊ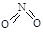 ��
��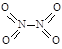 �� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��
�� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��
��2��ij�ָ��ܳ����ʹ������H2��Ĵ���Ͻ���MH��ʾ������ظ������ϣ�NiO(OH)���������ϣ�KOH��ҺΪ�������Һ�������ĵ缫��ӦΪ��MH��OH����e��= M��H2O����س�ŵ�ʱ���ܷ�ӦΪ��Ni(OH)2��M NiO(OH)��MH
NiO(OH)��MH
�� ��طŵ�ʱ�������ĵ缫��ӦʽΪ ��
�� ������ʱNi(OH)2ȫ��ת��ΪNiO(OH)����������罫��һ���缫����O2��ͬʱ��ɢ����һ���缫�����缫��Ӧ�����ģ���ʱ�����ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ���壮
��1�����������У�SO2����������SO3��2SO2��g��+O2��g�� 2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ1��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺
2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ1��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺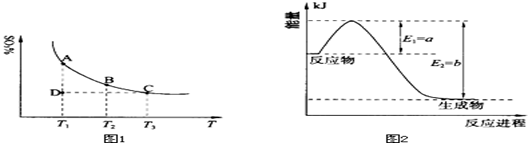
�ٺ��¡���ѹ�����£���Ӧ2SO2��g��+O2��g�� 2SO3��g����ƽ�⣬����ϵ��ͨ�뺤����ƽ�� �ƶ�������������ҡ���������
2SO3��g����ƽ�⣬����ϵ��ͨ�뺤����ƽ�� �ƶ�������������ҡ���������
�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1 K2�����������������=������ͬ��������Ӧ���е�״̬Dʱ��v�� v�������������������=������ͬ����
��2�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã�
����ͼ2��һ�����¶Ⱥ�ѹǿ����N2��H2��Ӧ����1molNH3�����������仯ʾ��ͼ����д����ҵ�ϳɰ����Ȼ�ѧ��Ӧ����ʽ�� ��
����H����ֵ�ú���ĸa��b�Ĵ���ʽ��ʾ��
�ڰ�������ˮ�õ���ˮ����25���£���a mol?L-1�İ�ˮ��b mol?L-1������������ϣ���Ӧ����Һǡ�������ԣ��ú�a��b�Ĵ���ʽ��ʾ����ˮ�ĵ���ƽ�ⳣ������ʽ ��
��3����֪25��CʱKsp[AgCl]=1.6��10-10mol2?L-2��Ksp[AgI]=1.5��10-16mol2?L-2������25���£���0.1L0.002mol?L-1��NaCl��Һ����μ���0.1L0.002mol?L-1��������Һ���а�ɫ�������ɣ��ӳ����ܽ�ƽ��ĽǶȽ��Ͳ���������ԭ���� ����Ӧ�����Һ�У���������0.1L0.002mol?L-1��NaI ��Һ�������������� �������������ԭ���ǣ������ӷ���ʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013���,�������������Ű�ҹ��ж�������������,����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
(1)����β����������Ҫԭ��Ϊ:2NO(g)+2CO(g) 2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��
2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��
�ݴ��ж�:
�ٸ÷�Ӧ�Ħ�H����0(�>����<��)��
����T2�¶���,0��2 s�ڵ�ƽ����Ӧ����v(N2)=����������
�۵��������������һ��ʱ,���������������ѧ��Ӧ���ʡ��������ı����S1>S2,����ͼ�л���c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н���,����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬������������(�����)�� 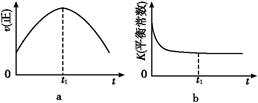
(2)ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
��úȼ�ղ���������������������,��CH4����ԭNOx�������������������Ⱦ��
����:
CH4(g)+2NO2(g) N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol
N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol
2NO2(g) N2O4(g) ��H2="-56.9" kJ/mol
N2O4(g) ��H2="-56.9" kJ/mol
д��CH4(g)����ԭN2O4(g)����N2(g)��H2O(g)���Ȼ�ѧ����ʽ:�� ��
�ڽ�ȼú�����Ķ�����̼��������,�ɴﵽ��̼�ŷŵ�Ŀ�ġ���ͼ��ͨ���˹��������,��CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦʽΪ�� �� 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�������Դ�����ʣ����ٻ�����Ⱦ���������Ž��ѳ����ȼ�ͼ״�����ɲ�ҵ������ͼ��ʾ��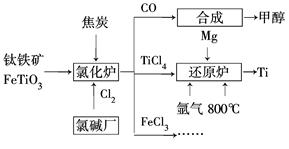
����д���пհס�
(1)����������Ȼ�¯ǰͨ����ȡϴ�ӡ����顢��ɡ�Ԥ�ȵ������������������ԭ���Ͻ��ͷ�������ã�_______________________________________
��֪�Ȼ�¯�������ͽ�̿�������������ʵ���֮��Ϊ7��6�����Ȼ�¯�л�ԭ���Ļ�ѧʽ��___________________________��
(2)��֪����Mg(s)��Cl2(g)=MgCl2(s)��H����641 kJ/mol
��2Mg(s)��TiCl4(s)= 2MgCl(s)��Ti(s)��H����512 kJ/mol
��Ti(s)��2Cl2(g)=TiCl4(s)����H��________��
(3)���ͨ�뻹ԭ¯�в������뷴Ӧ��ͨ�������������___________________________
(4)�Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ2CH3OH��3O2��4OH��=2CO32����6H2O���õ���������ϵĵ缫��ӦʽΪ_________________________________________��
����һ��ʱ������Һ��pH________(���С�����������䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013������������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
(1)����β����������Ҫԭ��Ϊ2NO(g)��2CO(g) 2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯������ͼ��ʾ��
2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯������ͼ��ʾ��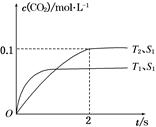
�ݴ��жϣ�
�ٸ÷�Ӧ�Ħ�H________0(�>����<��)
����T2�¶��£�0��2 s�ڵ�ƽ����Ӧ����v(N2)��______________________��
�۵��������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2������ͼ�л���c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����________(�����)��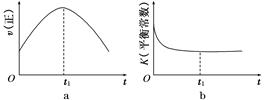
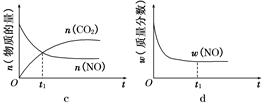
(2)ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
��úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
���磺CH4(g)��2NO2(g)=N2(g)��CO2(g)��2H2O(g)����H1����867 kJ/mol
2NO2(g)??N2O4(g)����H2����56.9 kJ/mol
д��CH4(g)����ԭN2O4(g)����N2(g)��CO2(g)��H2O(g)���Ȼ�ѧ����ʽ��________________________________________________________________________��
�ڽ�ȼú�����Ķ�����̼�������ã��ɴﵽ��̼�ŷŵ�
Ŀ�ġ���ͼ��ͨ���˹�������ã���CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦʽΪ__________________________��
�۳����£�0.1 mol��L��1��HCOONa��ҺpHΪ10����HCOOH�ĵ��볣��Ka��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʹ�������Դ����չ����̼���á�����Ϊ��ѧ���о�����Ҫ���⡣�������״������ʵ����ȼ�ϣ�������ȼ�ϵ�ء�
��1������ˮ����ת������H2����Ҫת����Ӧ���£�
CH4(g) + H2O(g) CO(g) + 3H2(g) ��H=+206��2 kJ��mol��1
CO(g) + 3H2(g) ��H=+206��2 kJ��mol��1
CH4(g) + 2H2O(g) CO2(g) + 4H2(g) ��H=+165��0 kJ��mol��1
CO2(g) + 4H2(g) ��H=+165��0 kJ��mol��1
������Ӧ����ԭ�����е�CO��ʹ�ϳɰ��Ĵ����ж��������ȥ����ҵ�ϳ����ô���������CO��ˮ������Ӧ�����׳�ȥ��CO2��ͬʱ���Ƶõ�����������ķ������˷�Ӧ��Ϊһ����̼�任��Ӧ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2�������״���ԭ��CO��H2��Դ�ڣ�CH4(g) + H2O(g)  CO(g) + 3H2(g) ��H>0
CO(g) + 3H2(g) ��H>0
��һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����A��B��C���㴦��Ӧƽ�ⳣ����KA��KB��KC���Ĵ�С��ϵΪ___________��(�<������>������="��" )��
��100��ʱ����1 mol CH4��2 mol H2Oͨ���ݻ�Ϊ1 L�Ķ����ܷ������У�������Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����__________
a�������������ܶȺ㶨
b����λʱ��������0��1 mol CH4ͬʱ����0��3 mol H2
c��������ѹǿ�㶨
d��3v��(CH4) = v��(H2)
��3��25��ʱ����20mL0��1mol/L������м���VmL0��1mol/LNaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����_____��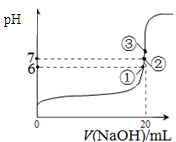
A��pH��3��HF��Һ��pH��11��NaF��Һ�У� ��ˮ�������c(H+)���
B���ٵ�ʱpH��6����ʱ��Һ�У�c(F��)��c(Na+)��9��9��10-7mol/L
C���ڵ�ʱ����Һ�е�c(F��)��c(Na+)
D���۵�ʱV��20mL����ʱ��Һ��c(Na+)��0��1mol/L
��4������������һֱ��Ϊ���ĺ�������ڡ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH��O��F��HFO��ˮ��Ӧ�õ�HF�ͻ�����A���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
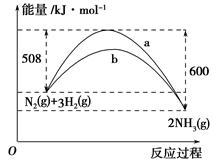
(1)�ٸ�������ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ___________________________��
�ڸ�����ͼ��ʾ������ж�����˵������ȷ����________��
| A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)=CO2(g)��H2(g)����H��41 kJ��mol��1 |
| B���÷�ӦΪ���ȷ�Ӧ |
| C���÷�ӦΪ���ȷ�Ӧ |
| D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ��mol��1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com