
NaCl��NaClO�����������¿ɷ�����Ӧ��ClO��+Cl��+2H+ = Cl2��+H2O��ijѧϰС�����о�����Һ(��Ҫ�ɷ�ΪNaCl��NaClO)�ı��������
��1��������Һ��NaClO�����տ����е�CO2����NaHCO3��HClO�����ʡ�д����ѧ��Ӧ����ʽ ��
��2��ȡ��������Һ�����Թ��У���������һ��Ũ�ȵ����ᣬ������ų���ͨ������װ�ü�������ijɷֿ����ж�����Һ�Ƿ���ʡ�
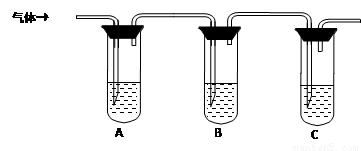
��ѡ�Լ���98%Ũ���ᡢ1%Ʒ����Һ��1.0 mol��L��1 KI-������Һ��1.0 mol��L��1NaOH������ʯ��ˮ������NaCl��Һ
���������ʵ�鷽����
|
�����Լ� |
Ԥ������ͽ��� |
|
�Թ�A�м������� �� �Թ�B�м�1%Ʒ����Һ�� �Թ�C�мӢ� �� |
��A����Һ����ɫ��B����Һ����ɫ��C����Һ����ǡ�������Һ���ֱ��ʣ� �� ������Һδ���ʣ� �� ������Һ��ȫ���ʡ� |
��3���õζ����ⶨ����Һ��NaClO��Ũ�ȡ�ʵ�鲽�����£�
����ȡ 25.00mL����Һ������ƿ�У����������a mol��L��1 Na2SO3��Һb mL��
�ڵζ���������c mol��L��1������KMnO4��Һװ�� ������ʽ���ʽ���ζ����У�KMnO4��ʣ���Na2SO3������Ӧ������Һ����ɫ���dz��ɫ���ұ��ְ�����ں�ɫ����ʱ��ֹͣ�ζ�����¼���ݡ��ظ��ζ�ʵ��2�Σ�ƽ����������KMnO4��Һv mL��
�ζ��������漰�ķ�Ӧ�У�NaClO + Na2SO3 = NaCl + Na2SO4 ��
2KMnO4 + 5Na2SO3+ 3H2SO4 = K2SO4 + 2MnSO4 + 5Na2SO4 + 3H2O
�ۼ��㡣����Һ��NaClO��Ũ��Ϊ mol��L��1���ú�a��b��c��v�Ĵ���ʽ��ʾ����
��16�֣�
��1��NaClO+CO2+H2O==NaHCO3+HClO ��3�֣�
��2��(��8��)
|
�����Լ� |
Ԥ������ͽ��� |
|
�� 1.0mol/L KI-������Һ��2�֣� �� ����ʯ��ˮ��2�֣� |
����A����Һ����ɫ��B����Һ����ɫ(�ޱ仯)��C����Һ�������(�ޱ仯)��������Һδ���ʣ�2�֣� ����A����Һ������ɫ(�ޱ仯)��B����Һ����ɫ(�ޱ仯)��C����Һ�����������Һ��ȫ���ʣ�2�֣� |
��3������ʽ ��2�֣�
�ۣ�2ab �C 5cv��/ 50 ��3�֣�
��������
�����������1�������⣬��Ҫ��Ӧ��ΪNaClO��CO2������ΪNaHCO3��HClO��������Ԫ�ػ��ϼ۱仯�����ݹ۲취��ƽ�ɵã�NaClO+CO2+H2O==NaHCO3+HClO����2������Һ�м�������ϡ����ʱ�����ܷ����ķ�ӦΪClO��+Cl��+2H+=Cl2��+H2O��HCO3��+H+=CO2��+H2O����ų���������ܺ���������������̼����A����Һ�������ɴ��ƶ��Թ�A�м����������1.0mol/LKI��������Һ����Ϊ������KI�ܷ����û���Ӧ���������û����ĵ��ʵ������B��Ʒ����Һ����ɫ��˵��������ȫ��A����Һ���ģ���C�б���ǣ�˵��������һ���ж�����̼���壬�Թ�C�м�����������ʯ��ˮ����Ϊ������̼������������Һ��Ӧ����̼��Ƴ�����ˮ���۸�����֪����ͽ����ƶϣ���A����Һ����ɫ��B����Һ����ɫ��C����Һ������ǣ�������Һδ���ʣ�����A����Һ������ɫ��B����Һ����ɫ��C����Һ����ǣ�������Һ��ȫ���ʣ���3���٢����Ը��������Һ�����ԣ����Ӧ��װ����ʽ�ζ����У������⣬����n=c•V�������������ܵ����ʵ���Ϊab��10��3mol��ÿ�εζ����ĵĸ������Ϊ cv��10��3mol������2KMnO4+5Na2SO3+3H2SO4=K2SO4+2MnSO4+5Na2SO4+3H2O�и����ʵ�ϵ��֮�ȵ������ʵ���֮�ȣ����������������������Ϊ5cv/2��10��3mol��������������������������Ϊ��ab��10��3��5cv/2��10��3��mol������NaClO+Na2SO3=NaCl+Na2SO4�и����ʵ�ϵ��֮�ȵ������ʵ���֮�ȣ����������Ϊ��ab��10��3��5cv/2��10��3��mol������c=n/V��V=0.025L����������Ƶ����ʵ���Ũ��Ϊ��ab��10��3��5cv/2��10��3��mol��0.025L =(2ab �C 5cv)/ 50mol/L��
���㣺���黯ѧʵ�鷽������ƺͲⶨ��Ʒ���ȵĻ�ѧ���㣬�漰����������Һ�������̼�ķ�Ӧԭ�������ʵ�鷽��̽������������Һ�Ƿ���ʡ��ζ��ܵ�ѡ�ⶨ����������Һ�����ʵ���Ũ�ȡ����ʵ����ڻ�ѧ����ʽ�е�Ӧ�õȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?���ڶ�ģ��NaCl��NaClO�����������¿ɷ�����Ӧ��ClO-+Cl-+2H+=Cl2��+H2O��ijѧϰС�����о�����Һ����Ҫ�ɷ�ΪNaCl��NaClO���ı��������
��2012?���ڶ�ģ��NaCl��NaClO�����������¿ɷ�����Ӧ��ClO-+Cl-+2H+=Cl2��+H2O��ijѧϰС�����о�����Һ����Ҫ�ɷ�ΪNaCl��NaClO���ı��������| �����Լ� | Ԥ������ͽ��� |
| �Թ�A�м������� 1.0mol/LK�� ����Һ 1.0mol/LK�� ������Һ �Թ�B�м�1%Ʒ����Һ�� �Թ�C�мӢ� ����ʯ��ˮ ����ʯ��ˮ �� |
��A����Һ����ɫ��B����Һ����ɫ��C����Һ����ǣ�������Һ���ֱ��ʣ� �� ��A����Һ����ɫ��B����Һ����ɫ ���ޱ仯����C����Һ������ǣ��ޱ仯������ ����Һδ���� ��A����Һ����ɫ��B����Һ����ɫ ������Һδ���ʣ����ޱ仯����C����Һ������ǣ��ޱ仯������ ����Һδ���� �� ��A����Һ������ɫ���ޱ仯����B���� Һ����ɫ���ޱ仯����C����Һ��������� ��Һ��ȫ���� ��A����Һ������ɫ���ޱ仯����B���� ������Һ��ȫ���ʣ�Һ����ɫ���ޱ仯����C����Һ��������� ��Һ��ȫ���� |
| (2ab-5vc) |
| 50 |
| (2ab-5vc) |
| 50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?������ģ��ij����Һ����Ҫ�ɷ�ΪNaCl��NaClO���ڿ�����������CO2�����ʣ���NaCl��NaClO�����������¿ɷ�����Ӧ��ClO-+Cl-+2H+=Cl2��+H2O��ijѧϰС����̽��������Һ�ı��������
��2013?������ģ��ij����Һ����Ҫ�ɷ�ΪNaCl��NaClO���ڿ�����������CO2�����ʣ���NaCl��NaClO�����������¿ɷ�����Ӧ��ClO-+Cl-+2H+=Cl2��+H2O��ijѧϰС����̽��������Һ�ı��������| �����Լ� | Ԥ������ͽ��� |
| �Թ�A�м����� �� �� ������ţ����Թ�B�м�1%Ʒ����Һ�� �Թ�C�м� �� �� ������ţ��� |
�� A����Һ����ɫ��B����Һ����ɫ��C����Һ����� A����Һ����ɫ��B����Һ����ɫ��C����Һ����� ����׳����� |
av1-
| ||
| 25 |
av1-
| ||
| 25 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭNaOH��Һ����������Ϊ10.0% | B��������Һ��Cl-�����ʵ���Ϊ0.25mol | C���μӷ�Ӧ�����������ʵ���Ϊ0.1mol | D�����������Ͳμӷ�Ӧ�����������ʵ���֮��Ϊ2�s3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com