
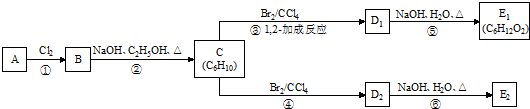
��16�֣���֪A��һ�����࣬����Է�����Ϊ56��1molA���������ȼ�պ�����4molCO2��
�ӻ�����A����������ת����ϵ��
����������Ϣ���ش��������⣺
��1��������A�ķ���ʽΪ ���ṹ��ʽΪ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ�� ��
���� ����Ӧ���ͣ���
��3����Ӧ�۵Ļ�ѧ����ʽΪ�� ��
���� ����Ӧ���ͣ���
��4��������Bһ�������¿��������������ӳɷ�Ӧ��1molB���������� molH2 ����д���÷�Ӧ��ѧ����ʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



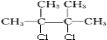 +2NaOH
+2NaOH| CH3CH2OH |
| �� |
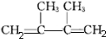 +2NaCl+2H2O
+2NaCl+2H2O +2NaOH
+2NaOH| CH3CH2OH |
| �� |
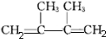 +2NaCl+2H2O
+2NaCl+2H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


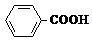 ��Ӧ�Ļ�ѧ����ʽ��
��Ӧ�Ļ�ѧ����ʽ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ��ɽ�и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��16�֣� ��֪A��һ�����࣬����Է�����Ϊ56��1molA���������ȼ�պ�����4molCO2��
�ӻ�����A����������ת����ϵ��

����������Ϣ���ش��������⣺
��1��������A�ķ���ʽΪ ���ṹ��ʽΪ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ�� ��
���� ����Ӧ���ͣ���
��3����Ӧ�۵Ļ�ѧ����ʽΪ�� ��
���� ����Ӧ���ͣ���
��4��������Bһ�������¿��������������ӳɷ�Ӧ��1molB���������� molH2 ����д���÷�Ӧ��ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ��㶫ʡ��ɽ���Ϻ�һ�и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��16�֣���֪A��һ �����࣬����Է�����Ϊ56��1molA���������ȼ�պ�����4molCO2��
�����࣬����Է�����Ϊ56��1molA���������ȼ�պ�����4molCO2��
�ӻ�����A����������ת����ϵ��
����������Ϣ���ش��������⣺
��1��������A�ķ���ʽΪ ���ṹ��ʽΪ ��
���ṹ��ʽΪ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ�� ��
���� ����Ӧ���ͣ���
��3����Ӧ�۵Ļ�ѧ����ʽΪ�� ��
���� ����Ӧ���ͣ���
��4��������Bһ�������¿��������������ӳɷ�Ӧ��1molB���������� molH2 ����д���÷�Ӧ��ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com