
Fe3O4����Ҫ�Ļ�ѧ�Լ���������������ȼ������ȡ����Ϊ�����Ϳ�ݵķ�����ͼ1����ȡ������������ϵ��װ�ã�Aװ��������ȡ������̼���壬��Ҫ�������ȶ������ٿɿء�
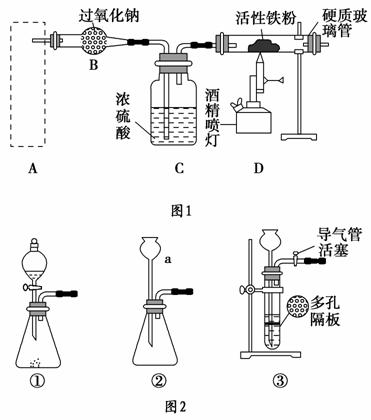
��ش��������⣺
(1)ͼ2������a��������________��
(2)������ĿҪ����ͼ2�����ѡ��________(�����)��ΪAװ�á�
(3)��ͼ2��װ�âٽ��������Լ��ķ�����________�����Һ©���м�����ˮ����һ�ᣬˮ�����µΣ�˵��װ�âٵ����������á�
(4)��Bװ���з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��_________________________ _______________________________________________________________________________________________________________________��
(5)������������������Ԥ�ȵ����۽Ӵ�ʱ����Ӳ�ʲ������н��۲쵽������������________________________________________________________��
(6)��Ӧһ��ʱ�������Ӳ�ʲ������еĹ����ĩ�������ܽ⣬ȡ������Һ���Թ��У�������۵⻯����Һ��û����ɫ���֣��Ʋ�����ĩ�г�Fe3O4�⣬һ����_______________________________________________________��
(7)�����۱���ȫ���ģ�ijͬѧΪȷ�����������У�2������ȡ�������������Թ��У�����������ϡ�����ܽ⡣
��д���ܽ���̷�����Ӧ�����ӷ���ʽ______________________ ________________________________________________________��
��д��ȷ����2���������Լ����ơ��������衢ʵ������ͽ���_______________________________________________________________��
���������⿼��Fe3O4���Ʊ��������ʼ���ȣ����ڿ��鿼�����������ʵ�鷽����������(1)ͼ2������aΪ����©����(2)ͼ2������װ��ֻ�Тۿ��Դﵽ���ٿɿص�Ŀ�ġ�(3)���װ�âٵ�������ʱ����ֹˮ�м�ס�ܣ�ȷ��װ�����ܷ�סһ����������塣(4)Bװ����Na2O2��CO2��Ӧ����Na2CO3��O2��(5)��Ӳ�ʲ�������������O2���ҷ�Ӧ�����л��Dz�����(6)�������KI��Һû�г�����ɫ��˵����Һ��û��Fe3�������������г�����Fe3O4�⣬��������ʣ�࣬Fe3O4�����������ɵ�Fe3����Fe��ԭΪFe2����(7)��Fe3O4����ϡ��������Fe3����Fe2���������ӷ���ʽΪFe3O4��8H��===2Fe3����Fe2����4H2O���ڼ�����Һ�к���Fe2��������K3[Fe(CN)6]��Һ����������Һ��
�𰸡�(1)����©����(2)�ۡ�(3)��ֹˮ�мн��ܡ�(4)2Na2O2��2CO2===2Na2CO3��O2
(5)����ȼ�գ��л������䡡(6)���ۡ�(7)��Fe3O4��8H��===2Fe3����Fe2����4H2O������С�Թ�ȡ������Һ����������μ���K3[Fe(CN)6]��Һ����������ɫ������˵����Fe2��(����С�Թ�ȡ�������������Һ�����뼸��������Һ������ɫ��dz��˵����Fe2������)


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ֵ���ͨ����ICl��IBr����Ϊ±�ػ���������ʺ�±�ص������ƣ����ǽ�ǿ������������������ʱICl��IBr�Կ���I2һ���������ڴֵ��м�������ѡ���е�һ�����ʺ��ٽ����������Ƶþ��⣬Ӧ����������� (����)��
A��H2O�� B��Zn��
C��KI�� D��KCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2.8 g Feȫ������һ��Ũ�ȡ�200 mL ��HNO3��Һ�У��õ���״���µ�����1.12 L����÷�Ӧ����Һ��pHΪ1������Ӧǰ����Һ����仯���Բ��ƣ��������й��ж���ȷ���� (����)��
A����Ӧ����Һ��c(NO )��0.80 mol��L��1
)��0.80 mol��L��1
B����Ӧ�����Һ�����ܽ�1.82 g Fe
C����ӦǰHNO3��Һ��Ũ��Ϊ1.0 mol��L��1
D��1.12 L������NO��NO2�Ļ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ͻ��ǽ��캽��ĸ����������ϡ�
(1)��ĸ�������������Ͻ����졣
����Ԫ�������ڱ��е�λ��Ϊ_________________________��
��ҵ������ԭ������������ȡ���ã���ȡ������ͨ�������Ϊ______________��
��Al��Mg�Ͻ�ǰ��NaOH��Һ����Al2O3Ĥ���仯ѧ����ʽΪ____________________�����ӹ�����ʹ�õı�����Ϊ__________________(�ѧʽ)��
(2)��ĸ�������Ϊ�Ͻ�֡�
�ٽ����ں�ˮ�з����ĵ绯ѧ��ʴ��ҪΪ___________________________ _____________________________________________��
�ں�ĸ�øֿ��ɵ�����ұ�����ɣ���������������Ϊ���躬������������Ϊ______________��
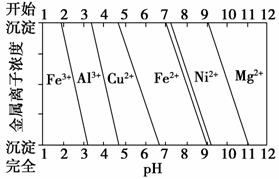
(3)��ĸ��������Ҫ��ͭ�Ͻ����졣
��80.0 g Cu��Al�Ͻ�������ȫ�ܽ���������ˮ�����˵ð�ɫ����39.0 g����Ͻ���Cu����������Ϊ________________________��
��Ϊ����ijͭ�Ͻ�ijɷ֣����Ὣ����ȫ�ܽ����NaOH��Һ����pH����pH��3.4ʱ��ʼ���ֳ������ֱ���pHΪ7.0��8.0ʱ���˳����������ͼ��Ϣ�ƶϸúϽ��г�ͭ��һ������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������KSCN(SCN���ǡ���±���ӡ�)��Һ���뵽�����ữ����������Һ�У���Һ��ɺ�ɫ�����ú�ɫ��Һ��Ϊ���ݣ���һ���м�������KMnO4��Һ����ɫ��ȥ��������һ����ͨ��SO2����ɫҲ��ȥ�������Ʋ�϶�����ȷ���� (����)��
A�����к�ɫ��ȥ��ԭ����KMnO4��SCN������
B�����к�ɫ��ȥ��ԭ����SO2��Fe3����ԭ��Fe2��
C�����к�ɫ��ȥ��ԭ����SO2��SCN����ԭ
D��SCN�����ʵ������¿�ʧȥ���ӱ�����Ϊ(SCN)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
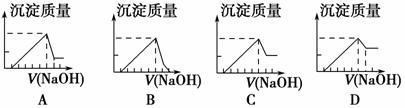
��һ��þ���Ͻ�����þ��������������8��9����������ϡH2SO4ʹ����ȫ�ܽ���ټ���NaOH��Һ�����ɳ�����������NaOH��Һ����仯����������ͼ��������ȷ���� (����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮAlCl3���������������л��ϳɵĴ����ȡ���ҵ����������(Al2O3��Fe2O3)Ϊԭ���Ʊ���ˮAlCl3�Ĺ����������¡�
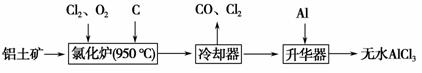
(1)�Ȼ�¯��Al2O3��Cl2��C��Ӧ�Ļ�ѧ����ʽΪ_____________________ _________________________________________________________��
(2)��Na2SO3��Һ�ɳ�ȥ��ȴ���ų���β���е�Cl2���˷�Ӧ�����ӷ���ʽΪ_______________________________________________________________��
(3)����������Ҫ����AlCl3��FeCl3�����������Al����������___________ _____________________________________________________________��
(4)Ϊ�ⶨ�Ƶõ���ˮAlCl3��Ʒ(������FeCl3)�Ĵ��ȣ���ȡ16.25 g��ˮAlCl3��Ʒ�����ڹ�����NaOH��Һ�У����˳�����������ᆳϴ�ӡ����ա���ȴ�����أ���������Ϊ0.32 g��
��д���������ӹ������漰�����ӷ���ʽ��__________________________��__________________________��
��AlCl3��Ʒ�Ĵ���Ϊ__________��
(5)��ҵ����һ��������Ϊԭ���Ʊ���ˮAlCl3�Ĺ����У����һ������AlCl3��6H2O��ˮ�Ʊ���ˮAlCl3��ʵ����һ���ķ�����__________________ ______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(NH4)2PtCl6�������ȷֽ⣬���ɵ������Ȼ��⡢�Ȼ�狀ͽ��������ڴ˷ֽⷴӦ�У����������뻹ԭ��������ʵ���֮���� (����)��
A��2��3 B��3��2
C��4��3 D��1��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A��SiO2����������������κ��ᡢˮ��Ӧ��������NaOH��Һ��Ӧ
B��������ˮ�����ԣ������еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ
C��CO2��SO2��NO2������ˮ����������
D��Fe3O4���ᷴӦʱ������FeԪ�صļ�̬����Ϊ+2��+3��+2��+3�۵Ļ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com