
��֪��ӦCu(s) + 2Ag+(aq) = Cu2+(aq) + 2Ag(s)Ϊһ�Է����е�������ԭ��Ӧ��������Ƴ���ͼ��ʾԭ��ء�����˵���в���ȷ����
A���缫X�Ǹ�������缫��ӦΪCu��2e- �� Cu2+
B�����缫������С��Y��Һ��c(Ag+)����
C��ʵ�������ȡ�����ţ�ԭ����Լ�������
D����X�缫��������0.64 gʱ�����·����0.02 mol����ת��
 ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д� ����5��2���ϵ�д�
����5��2���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʱȽ��У���ȷ���� �� ��
�����ԣ�HClO4>HBrO4>HIO4 �ڼ��ԣ�Ba(OH)2>Mg(OH)2>Be(OH)2
�������ԣ�F>C>O �ܻ�ԭ�ԣ�Cl<S<Si
����̬�⻯���ȶ��ԣ�HF��HCl��H2S
A���٢ڢ� B���ڢۢ� C���٢ڢܢ� D���٢ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪25 �桢101 kPa�£�ʯī�����ʯȼ�յĻ�ѧ����ʽ�ֱ�Ϊ��C(ʯī) + O2(g) = CO2(g)��1 moL C(ʯī) ��ȫȼ�շ���393.51 kJ��C(���ʯ) + O2(g) = CO2(g)��1 moL C(���ʯ) ��ȫȼ�� ���� 395.41 kJ���ݴ��������õ������н����У���ȷ���ǣ� ����
A�����ʯ��ʯī�ȶ� B��ʯīת��Ϊ���ʯ�������仯
C��ʯī�������Ƚ��ʯ�������� D����ʯī�Ʊ����ʯһ�������ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±������ڱ��е�һ����,����A��I�����ڱ��е�λ��,��(1)~ (4)С����Ԫ�ط��Ż�ѧʽ�ش𣬣�5������8��С�ⰴ��ĿҪ��ش�
| �� ���� | I A | �� A | �� A | �� A | �� A | �� A | �� A | O |
| 1 | A | |||||||
| 2 | D | E | G | I | ||||
| 3 | B | C | F | H |
(1)����Ԫ��,��ѧ��������õ��� ,ֻ�и��۶������۵��� ,��������ǿ�ĵ����� ,��ԭ����ǿ�ĵ����� .
(2)����������ˮ���������ǿ���� ,������ǿ���� ,�����Ե��� .
(3)A�ֱ���D��E��F��G��H�γɵĻ�������,���ȶ��� ��
(4)��B��C��E��F��G��H��,ԭ�Ӱ뾶������ ��
��5��A��D��ɻ�����ĵ���ʽ ��
��6��A��E��ɻ�����Ļ�ѧʽ ��
��7���õ���ʽ��ʾB��H��ɻ�������γɹ��̣�
��
��8��B������������ˮ�����C��������������Ӧ�����ӷ���ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A�����������ʶ��������绯ѧ��ʴ�����ԺϽ���ʴ
B��ԭ��ط�Ӧ�ǵ��½�����ʴ����Ҫԭ�ʲ����������������ĸ�ʴ
C������������ѧ��ʴ���ǵ绯ѧ��ʴ����Ҫ�������ڽ����Ĵ��Ȳ�ͬ
D�������������͵ĸ�ʴ����ʵ�ʶ��ǽ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼװ���У�U����Ϊ��īˮ��a��b�Թ��ڷֱ�ʢ��ʳ��ˮ��ϡ������Һ�������������飬����һ��ʱ�䡣�����й������������
A���������е�̼��ԭ��ص�����
B�����Թ�����ͬ�ĵ缫��Ӧʽ�ǣ�Fe��2e-= Fe2+
C����īˮ�����ߵ�Һ���Ϊ����Ҹ�
D��a�Թ��з�����������ʴ��b�Թ��з��������ⸯʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�ӦaA(g)��bB(g)  pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����
(1)�÷�Ӧ���淴Ӧ��________�ȷ�Ӧ����a��b________p(�>����<������)��
(2)��ѹʱ��A����������________(�������С�����䡱����ͬ)������Ӧ����________��
(3)������B(�������)����A��ת����________��B��ת����________��
(4)�������¶ȣ���ƽ��ʱ��B��C��Ũ��֮��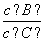 ��________��
��________��
(5)�����������ƽ��ʱ��������������ʵ���________��
(6)��B����ɫ����,A��C��Ϊ��ɫ����,�����C(�������)ʱ��������ɫ________����ά�������������ѹǿ����,��������ʱ,��������ɫ________��(���dz����������䡱)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
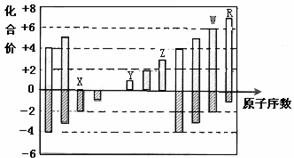
��ͼ�Dz��ֶ�����Ԫ�صij������ϼ���ԭ�������Ĺ�ϵͼ������˵����ȷ����
A.ԭ�Ӱ뾶��Z��Y��X
B.��̬�⻯����ȶ��ԣ�R��W
C. W�����������ΪWO2
D.Yʧ����������Zǿ��Y�ĵ����ܴ�Z������Һ���û���Z�ĵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������
A��6Li�� 7Li�ĵ�������ȣ�������Ҳ���
B��1H�� 2H�Dz�ͬ�ĺ��أ����ǵ����������
C��14C�� 14N����������ȣ����ǵ�����������
D��13C�� 14C����ͬһ��Ԫ�أ����ǻ�Ϊͬλ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com