
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� �� ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����ͼ1-4-25��ʾģ��д�����Ľṹ��ʽ��____________________________________��
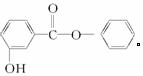
ͼ1-4-25
(2)����ˮ�⡢���롢�ᴿ�ɵõ������ı��Ӻ�ˮ����(���ǻ�������)�������һ��������˵�����ӡ�̼�ᡢˮ���������������ǿ(�û�ѧ����ʽ��ʾ)��
(3)ͬʱ���������ĸ�Ҫ���ˮ�����ͬ���칹�干��__________�֡�
�ٺ��б�����
���ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��
����ϡNaOH��Һ�У�1 mol��ͬ���칹������2 mol NaOH������Ӧ��
��ֻ����������һ�ȴ����
(4)��(3)ȷ����ͬ���칹������ѡһ�֣�ָ��Ϊͼ1-4-26��ʾ�Ŀ�ͼ�е�A��
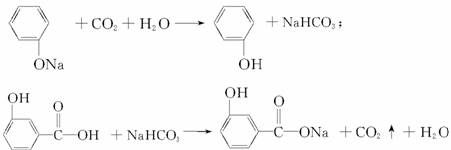
ͼ1-4-26
д������������Ӧ�Ļ�ѧ����ʽ(�л����ýṹ��ʽ��ʾ)����ָ����Ӧ�ķ�Ӧ���͡�
��A��B
��B+D��E
(5)����ˮ����ͱ��ӵĻ������ǵ����ʵ���֮��Ϊn mol���û������ȫȼ������a L O2��������b g H2O��c L CO2(���������Ϊ��״���µ����)��
�ٷֱ�д��ˮ����ͱ�����ȫȼ�յĻ�ѧ����ʽ(�л�����÷���ʽ��ʾ)
����������ˮ��������ʵ���Ϊx mol���г�x�ļ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� ��
 ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡâ�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� ��
 ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com