
һ�ֺ�����ﮡ��ܵ����͵��Ӳ��ϣ������в����ķ��������ɹۣ������е����Խ�����������ʽ���ڣ�����Co2O3��CoO����ʽ���ڣ������������ĵ����˫�棻﮻��������С�(��֪Co2O3�������ԣ�Cl2��������)
�ӷ����л���������(CoO)�Ĺ����������£�
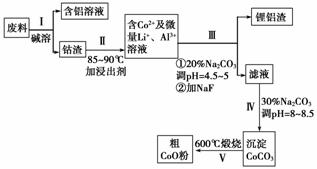
(1)���̢��в���NaOH��Һ�ܳ������е�Al����Ӧ�����ӷ���ʽΪ____________��
(2)���̢��м���ϡH2SO4�ữ���ټ���Na2S2O3��Һ�����ܡ�������������ʵĻ�ѧ��Ӧ����ʽΪ(������ֻ��һ�����)________����ʵ����ģ�ҵ����ʱ��Ҳ������������ܣ���ʵ�ʹ�ҵ�����в������ᣬ�����������������ܵ���Ҫԭ��________________________________________________________________________
________________________________________________________________________��
(3)���̢�õ����������Ҫ�ɷ���LiF��Al(OH)3��̼������Һ�ڲ���Al(OH)3ʱ����Ҫ���ã���д���÷�Ӧ�����ӷ���ʽ��________________________________________
(4)̼������Һ�ڹ��̢�͢�����������������ͬ����д���ڹ��̢������������________________________________________________________________________��
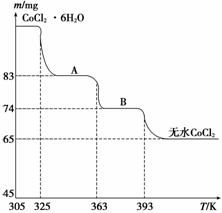
(5)CoO��������ɵ÷ۺ�ɫ��CoCl2��Һ��CoCl2���ᾧˮ��Ŀ��ͬ�����ֲ�ͬ��ɫ��������ɫ����ˮCoCl2��ˮ��ɫ��һ���ʿ��Ƴɱ�ɫˮ�������īˮ����ͼ�Ƿۺ�ɫ��CoCl2��6H2O�������ȷֽ�ʱ��ʣ������������¶ȱ仯�����ߣ�����A�Ļ�ѧʽ��________________________________________________________________________��
�𰸡�(1)2Al��2OH����6H2O===2[Al(OH)4]����3H2��
(2)4Co2O3��CoO��Na2S2O3��11H2SO4===12CoSO4��Na2SO4��11H2O
Co2O3��CoO�������������Cl2����Ⱦ����
(3)2Al3����3CO ��3H2O===2Al(OH)3����3CO2��
��3H2O===2Al(OH)3����3CO2��
(4)����pH���ṩCO ��ʹCo2������ΪCoCO3
��ʹCo2������ΪCoCO3
(5)CoCl2��2H2O
������(2)������CoΪ��3�ۺͣ�2�ۣ��ɹ��������й��̢����Һֻ����Co2�������֪Co3������S2O ����ԭ����ΪCo2�����ɲ�����ֻ��һ�������֪��������ֻ��SO
����ԭ����ΪCo2�����ɲ�����ֻ��һ�������֪��������ֻ��SO ��(3)Al3����CO
��(3)Al3����CO ���Է���˫ˮ������Al(OH)3������(4)�ɹ��̢�����ʾ����Na2CO3�����pH������CoCO3�����ɻ�����ڹ����е����á�(5)����ˮCoCl2��֪�����ʵ���Ϊn(CoCl2)��65��10��3 g��130 g��mol��1��5��10��4 mol��A�к���ˮ�����ʵ���Ϊn(H2O)��(83��65)��10��3 g��18 g��mol��1��1��10��3 mol����n(CoCl2)��n(H2O)��1��2��������AΪCoCl2��2H2O��
���Է���˫ˮ������Al(OH)3������(4)�ɹ��̢�����ʾ����Na2CO3�����pH������CoCO3�����ɻ�����ڹ����е����á�(5)����ˮCoCl2��֪�����ʵ���Ϊn(CoCl2)��65��10��3 g��130 g��mol��1��5��10��4 mol��A�к���ˮ�����ʵ���Ϊn(H2O)��(83��65)��10��3 g��18 g��mol��1��1��10��3 mol����n(CoCl2)��n(H2O)��1��2��������AΪCoCl2��2H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڢ���ϩ�ڱ����Ҵ�������������ǵ��л������������ȷ����
A�����������Ƶ�Cu(OH)2����Һ����ۢܢ� Bֻ�Т�(3���ܷ���ȡ����Ӧ
Cֻ�Т٢ۢ���ʹ����KMnO4��Һ��ɫ D��һ�������£��ݿ���ת��Ϊ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�����ʳ������Ա仯�ľ��������� �� ��
A��Ԫ��ԭ�Ӱ뾶��С�������Ա仯 B��Ԫ�ص����ԭ���������ε���
C��Ԫ��ԭ�Ӻ�������Ų��������Ա仯 D��Ԫ�ص���������ϼ۳������Ա仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�����ȼ���������ڹ��������·�Ӧ�Ļ�ѧ����ʽ
______________________________________________���û�ѧ��Ӧ�ķ�Ӧ����Ϊ
______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ͻ��봿�����ƳɵĽ���������ȣ��ŵ��� (����)
�ٺϽ��Ӳ��һ������ĸ��ɷֽ����Ĵ�
��һ��أ��Ͻ���۵�����ĸ��ɷֽ����ĸ���
�۸ı�ԭ�ϵ���ȡ��ı����ɺϽ���������õ��в�ͬ���ܵĺϽ�
�ܺϽ�ȴ������ĵ����Ը�ǿ
�ݺϽ�ȴ�������Ӧ�÷�Χ���㷺
A���ڢۢ� B���٢ڢۢ�
C���٢ڢ� D���٢ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ճ������Լ����������Ź㷺��Ӧ�á����й��ڽ�����һЩ˵������ȷ����
(����)
A���Ͻ����������ɷֽ��������ʲ���ȫ��ͬ
B����ҵ�Ͻ���Mg��Cu�������Ȼ�ԭ���Ƶõ�
C������ұ���ı����ǽ��������ӵõ����ӱ�ɽ���ԭ��
D��Խ���õĽ���Խ��ұ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ν�Ͻ𣬾��Dz�ͬ�ֽ���(Ҳ����һЩ�ǽ���)���ۻ�״̬���γɵ�һ���ۺ���±�Ϊ���ֽ������ۡ��е㣺
| Na | Cu | Al | Fe | |
| �۵�(��) | 97.5 | 1 083 | 660 | 1 535 |
| �е�(��) | 883 | 2 595 | 2 200 | 3 000 |
�������������ж����в����γɺϽ���� (����)
A��Cu��Al B��Fe��Cu
C��Fe ��Na D��Al��Na
��Na D��Al��Na
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�pH��3��HA��ҺV1 mL��pH��11��KOH��ҺV2 mL��ϣ�������˵����ȷ����(����)
A����V1��V2����Ӧ����Һ��pHһ������7
B������Ӧ����Һ�����ԣ���V1һ��С��V2
C������Ӧ����Һ�����ԣ���V1һ������V2
D������Ӧ����Һ�����ԣ�����Һ��һ������c(H��)��c(OH��)��2��10��7 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£�ʯ��������Һ�д�������ƽ�⣺Ca(OH)2(s) Ca2��(aq)��2OH��(aq)������һ������ʯ��������Һ�м���������ʯ��ʱ������˵������ȷ����
Ca2��(aq)��2OH��(aq)������һ������ʯ��������Һ�м���������ʯ��ʱ������˵������ȷ����
A.��Һ��Ca2����Ŀ���١� B.��Һ��c(Ca2��)����
C.��Һ��pH������ D.��Һ�����ʵ�������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com