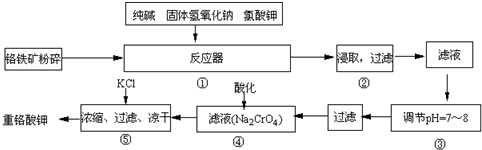
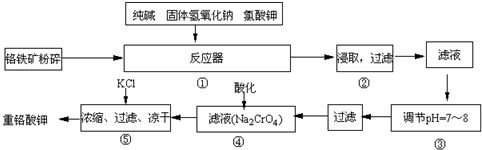
��2009?÷��ģ�⣩�ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO?Cr
2O
3��������Ҫ�Ƕ����������������Ϊԭ��������ʵ����ģ�ҵ���ø�������K
2Cr
2O
7����Ҫ�������£��漰����Ҫ��Ӧ�ǣ�
6FeO?Cr
2O
3+24NaOH+7KClO
312Na
2CrO
4+3Fe
2O
3+7KCl+12H
2O
�Իش���������
��1��ָ��
24Cr�����ڱ��е�λ����
�������ڵڢ�B
�������ڵڢ�B
��
��2��NaFeO
2��ǿ��ˮ�⣬�ڲ��������ɳ�������ȥ��д���÷�Ӧ�Ļ�ѧ����ʽ��
NaFeO2+2H2O=Fe��OH��3��+NaOH
NaFeO2+2H2O=Fe��OH��3��+NaOH
��
��3�������۵���pH=7��8��ԭ����
������Һ�ڹ����ƺ�ƫ�����Ʒ���ˮ�⣬SiO32-+H2O?HSiO3-+OH-��HSiO3-+H2O?H2SiO3+OH-��AlO2-+H2O?Al��OH��3+OH-������pHֵ������ƽ��������Ӧ�����ƶ�����pH����7��8ʱ��ʹ����ˮ����ȫ��
������Һ�ڹ����ƺ�ƫ�����Ʒ���ˮ�⣬SiO32-+H2O?HSiO3-+OH-��HSiO3-+H2O?H2SiO3+OH-��AlO2-+H2O?Al��OH��3+OH-������pHֵ������ƽ��������Ӧ�����ƶ�����pH����7��8ʱ��ʹ����ˮ����ȫ��
�����ü�Ҫ�����ֺ����ӷ���ʽ˵����
�±��Ǹ��ᣨH
2CrO
4����Һ�����ӵ������pH�Ĺ�ϵ��
| pH |
C��CrO42-��mol/L |
C��HCrO4-��mol/L |
C��Cr2O72-��mol/L |
C��H2CrO4��mol/L |
| 4 |
0.0003 |
0.104 |
0.448 |
0 |
| 5 |
0.0033 |
0.103 |
0.447 |
0 |
| 6 |
0.0319 |
0.0999 |
0.437 |
0 |
| 7 |
0.2745 |
0.086 |
0.3195 |
0 |
| 8 |
0.902 |
0.0282 |
0.0347 |
0 |
| 9 |
0.996 |
0.0031 |
0.0004 |
0 |
��4���������ữ��Ŀ����
�ữʹCrO42-ת��ΪCr2O72-
�ữʹCrO42-ת��ΪCr2O72-
��
��5���ڸ��ᣨH
2CrO
4����Һ�У������CrO
42-�ĵ���ƽ�ⳣ��ΪK
2�������Cr
2O
72-�ĵ���ƽ�ⳣ��ΪK
3����K
2��
��
K
3�������������
��6���ϱ��и�������ʵ���Ũ��Ϊ
1.00mol/L
1.00mol/L
������ȷ��С�����2λ��

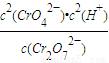 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��