��ŨCaCl
2��Һ��ͨ��NH
3��CO
2�������Ƶ�����̼��ƣ���ͼ��ʾA-EΪʵ���ҳ���������װ�ã����̶ֹ��г�װ����ȥ���������Ҫ��ش����⣮

��1��ʵ��������NH
4Cl����ʯ�����Լ�����ȡ���ռ������NH
3������ѡ����������װ���е�
BDE
BDE
����װ����ţ�����Ҫ��ȡ���ռ������CO
2����ѡ��װ�ò��������������Ӹ������ӿ�
acdh
acdh
��
��2������A�ķ�Һ©���ڸļ�Ũ��ˮ��Բ����ƿ�ڼ�NaOH���壬Ҳ����ȡ�����������װ��A���ܲ���������ԭ��
������������ˮ�ų������ȣ��¶����ߣ�ʹ�����ܽ�ȼ�С���ų�������������ˮ����ʹ���ų����������Ƶ������OH-�����˰�ˮ��OH-Ũ�ȣ���ʹ��ˮ����ƽ�����ƣ����°����ų�
������������ˮ�ų������ȣ��¶����ߣ�ʹ�����ܽ�ȼ�С���ų�������������ˮ����ʹ���ų����������Ƶ������OH-�����˰�ˮ��OH-Ũ�ȣ���ʹ��ˮ����ƽ�����ƣ����°����ų�
��
��3����ŨCaCl
2��Һ��ͨ��NH
3��CO
2��������̼���ʱ��Ӧ��ͨ���������
NH3
NH3
����ʵ��������а����ݳ���Ӧѡ������
b
b
װ�û��գ�����ţ���

д��������̼��ƵĻ�ѧ����ʽ
CaCl2+CO2+2NH3+H2O=CaCO3+2NH4Cl
CaCl2+CO2+2NH3+H2O=CaCO3+2NH4Cl
��
��4������Ƽ�ʵ�鷽�����ж�����̼�����Ʒ�����Ƿ�Ϊ����
ȡ������Ʒ��ˮ����γɷ�ɢϵ����һ�������䣬������һ��������ͨ·������������������
ȡ������Ʒ��ˮ����γɷ�ɢϵ����һ�������䣬������һ��������ͨ·������������������
��

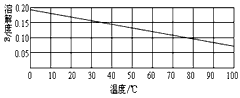
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

