
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL��1mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL��1mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У�| 1000��w |
| M |
| n |
| V |
| 1000��1.84��98% |
| 98 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
89 38 |
90 38 |
| A�������� | B�������� |
| C�������� | D�����ԭ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��
| ||||
| B��SO2��SO3 | ||||
| C��CH4��C4H10 | ||||
| D��CO��NH2��2��NH4CNO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1mol E���������������ã��ڱ�״�����ܲ���33.6L H2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ��
��A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1mol E���������������ã��ڱ�״�����ܲ���33.6L H2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
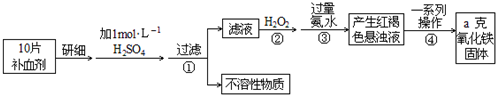
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
| �е�/�� | 57.7 | 12.8 | - | 315 | - |
| �۵�/�� | -70.0 | -107.2 | - | - | - |
| �����¶�/�� | - | - | 180 | 300 | 162 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.1mol?L-1 NaHCO3��Һ�У�c��H+��+2c��H2CO3��=2c��CO32-��+c��OH-�� |
| B��0.1mol?L-1 ��NH4��2Fe��SO4��2��Һ�У�c��SO42-��=c��NH4+����c��Fe2+����c��H+����c��OH-�� |
| C���������ơ���������Һ��Ϻ���Һ�����ԣ����Ϻ����Һ�У�c��Na+����c��Cl-�� |
| D��pH��ȵĢ�CH3COONa ��C6H5ONa ��Na2CO3 ��NaOH������Һ�����ʵ���Ũ�ȴ�С���٣��ڣ��ۣ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com