
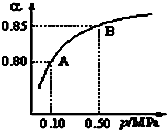
���� ��1���¶Ȳ��䣬��ƽ�ⳣ��K���䣻
��2����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g������H=-574kJ/mol
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g������H=-1160kJ/mol
���ø�˹���ɽ�$\frac{��+��}{2}$�ɵã�CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g�����Դ˼��㷴Ӧ�ȣ����Ԫ�صĻ��ϼ۱仯����ת�Ƶĵ�������
��3�����ݳ����ܽ�ƽ��CrO42-+Ba2+?BaCrO4��Ksp��BaCrO4��=C��CrO42-����C��Ba2+��=1.2��10-10���ɵ�C��Ba2+����
��� �⣺��1��ƽ�ⳣ��ֻ���¶ȵ�Ӱ�죬�¶Ȳ��䣬��ѹǿ���䣬ƽ��״̬��A�䵽Bʱ����k��A��=k��B����
�ʴ�Ϊ��=��
��2����֪����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g������H=-574kJ/mol
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g������H=-1160kJ/mol
���ø�˹���ɽ�$\frac{��+��}{2}$�ɵã�CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g������H=-867kJ/mol
n��CH4��=$\frac{4.48L}{22.4L/mol}$=0.2mol��
����������ת�Ƶĵ�������Ϊ��0.20mol��8NA=1.60NA��
�ų�������Ϊ��0.2mol��867kJ/mol=173.4kJ��
�ʴ�Ϊ��1.60NA����1.6NA����173.4��
��3��CrO42-+Ba2+?BaCrO4
5.0��10-7mol•L-1 C��Ba2+��
Ksp��BaCrO4��=C��CrO42-����C��Ba2+��=5.0��10-7��C��Ba2+��=1.2��10-10��
C��Ba2+��=2.4��10-4mol/L��
�ʴ�Ϊ��2.4��10-4��
���� ���⿼�黯ѧƽ�⼰��ѧ��Ӧ�������仯���ܶȻ������ļ����������Ŀ�Ѷ��еȣ������״���Ϊ��ѧƽ�ⳣ���ļ��㣬ע�������̵İ��գ�


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѿ�Ǽ���ˮ�������ܷ���������Ӧ | |
| B�� | ����ֽ��������ά����Ҫ�ɷֶ�����ά�� | |
| C�� | ���ۡ�ţ�͡������ʶ�����Ȼ�߷��ӻ����� | |
| D�� | ���ʵ���֬�����ŵ�������ζ����������֬������ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩʹ���Ը��������Һ��ɫ | |
| B�� | ����������ˮ�У���ˮ��ӽ���ɫ | |
| C�� | ������������Ϲ��գ����������ɫ��dz | |
| D�� | ��ϩʹ������Ȼ�̼��Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
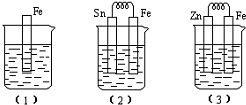
 ���ǶԱ�����ʶ��һ��������Ĺ��̣�
���ǶԱ�����ʶ��һ��������Ĺ��̣�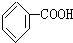 ����ʯ�ҵĻ����õ�Һ�壬����Ϊ����д������������NaOH�������ɱ��Ļ�ѧ����ʽ��
����ʯ�ҵĻ����õ�Һ�壬����Ϊ����д������������NaOH�������ɱ��Ļ�ѧ����ʽ��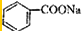 +NaOH$��_{��}^{������}$Na2CO3+
+NaOH$��_{��}^{������}$Na2CO3+ ��
��
 ����
�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
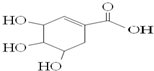
| A�� | �ɷ����ӳɺ�ȡ����Ӧ | |
| B�� | ����ʽΪC7H6O5 | |
| C�� | �����к���2�ֹ����� | |
| D�� | ��ˮ��Һ���Ȼ����ǻ����ܵ����H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �屽 | B�� | �Ҵ� | C�� | ���Ȼ�̼ | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �������ӣ�����Ũ��ˮ������ | |
| B�� | �������������ᣩ�������Ҵ���Ũ���Ტ���� | |
| C�� | �Ҵ���ˮ����������ʯ�ң����� | |
| D�� | ������Һ�������ǣ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
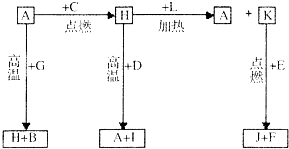 A��B��C��D��E��F�dz������ʣ�����A���������Ӧ����㷺�Ľ�����D�ǵؿ��к������Ľ���Ԫ�أ�DԪ�غ�EԪ�������ڱ������ڣ�G��H��I��J��K��L�dz������������G�ڳ���������ɫ��ζ��Һ�壬H�Ǻ�ɫ���壮������������ת����ϵ��ͼ��ʾ����ش��������⣺
A��B��C��D��E��F�dz������ʣ�����A���������Ӧ����㷺�Ľ�����D�ǵؿ��к������Ľ���Ԫ�أ�DԪ�غ�EԪ�������ڱ������ڣ�G��H��I��J��K��L�dz������������G�ڳ���������ɫ��ζ��Һ�壬H�Ǻ�ɫ���壮������������ת����ϵ��ͼ��ʾ����ش��������⣺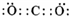 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com