
����Ŀ��(1)�ڢ�![]() Li����ʯī����C60����
Li����ʯī����C60����![]() Mg �� CH3CH2OH ��
Mg �� CH3CH2OH ��![]() C ��
C ��![]() Li �� CH3OCH3 �У�____��Ϊͬλ�أ� ____��Ϊͬ���칹�壻___��Ϊͬ��������(�����)
Li �� CH3OCH3 �У�____��Ϊͬλ�أ� ____��Ϊͬ���칹�壻___��Ϊͬ��������(�����)
(2)���Т�CaCl2 �ڽ��ʯ ��NH4Cl ��Na2SO4 �ݱ� ���������ʣ�������Ҫ��ش�
���ۻ�ʱ����Ҫ�ƻ���ѧ������___________���۵���ߵ���_______��(�����)
��ֻ�������Ӽ���������______�������Է��Ӽ���������ϵ���______��(�����)
(3)д���������ʵĵ���ʽ
��H2O______
��NaOH______
��NH3______
���𰸡��٢� �ݢ� �ڢ� �� �� �� �� ![]()
![]()
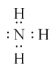
��������
(1)��������ͬ����������ͬ��ԭ�ӻ�Ϊͬλ�أ�����ʽ��ͬ���ṹ��ͬ���л��ﻥΪͬ���칹�壻ͬ��Ԫ����ɵIJ�ͬ���ʻ�Ϊͬ�������壻
(2)��CaCl2�����Ӿ��壻�ڽ��ʯ��ԭ�Ӿ��壻 ��NH4Cl�����Ӿ��壻��Na2SO4�����Ӿ��壻�ݱ��Ƿ��Ӿ��壻
(3)��H2O�ǹ��ۻ������NaOH�����ӻ������NH3�ǹ��ۻ����
(1)��![]() Li�� ��
Li�� ��![]() Li����������ͬ����������ͬ��ԭ�ӣ��١���Ϊͬλ�أ�CH3CH2OH��CH3OCH3�ķ���ʽ����C2H6O���ṹ��ͬ���ݡ��Ϊͬ���칹�壻ʯī��C60������CԪ����ɵĵ��ʣ��ڡ��ۻ�Ϊͬ�������壻
Li����������ͬ����������ͬ��ԭ�ӣ��١���Ϊͬλ�أ�CH3CH2OH��CH3OCH3�ķ���ʽ����C2H6O���ṹ��ͬ���ݡ��Ϊͬ���칹�壻ʯī��C60������CԪ����ɵĵ��ʣ��ڡ��ۻ�Ϊͬ�������壻
(2)�ٷ��Ӿ����ۻ�ʱ�ƻ����Ӽ����������ۻ�ʱ����Ҫ�ƻ���ѧ�����DZ���ѡ�ݣ�ԭ�Ӿ����ۻ����ƻ����ۼ���ԭ�Ӿ�����۵�ߣ��۵���ߵ��ǽ��ʯ��ѡ�ڣ�
��CaCl2�����Ӿ��壬ֻ�������Ӽ���ѡ�٣����Ӿ����Է��Ӽ���������ϣ��Է��Ӽ���������ϵľ����DZ���ѡ�ݣ�
(3)��H2O�ǹ��ۻ��������ʽ��![]() ��
��
��NaOH�����ӻ��������ʽ��![]() ��
��
��NH3�ǹ��ۻ��������ʽ��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1������������̬������ʵ��������Ȼ�ĺ�г������
������������Ӿ�����ЧӦ����__________������ĸ����
a.ֲ������ b.ȼú��ů c.��������
�����з��Ρ���ɫ��Ⱦ������ȷ������_____________������ĸ����
a.ʹ�ÿɽ������� b.¶����շϾ����� c.ֱ������Ͼ�����
��Ϊ���������Ⱦ����������ѽ�ֹȼ���̻�������ֹȼ���̻����ı�ʶ��_____������ĸ����

��2������ʹ�û�ѧ֪ʶ��������ǵ�����������
ijƷ������ijɷ��и��͡�ɽ����ء������Ƶȡ�
������������ɷ��У����ڷ���������_______________��
�ڸ��͵Ľṹ��ʽΪ____________����֬ˮ������ɸ��ͺ�_____________��
�۷�����(NaF)���������е��ǻ������[Ca5(PO4)3OH]��Ӧ�����ɸ����ܵķ������[Ca5(PO4)3F]���Ӷ��ﵽ����ȣ�ݵ�Ŀ�ġ�д���÷�Ӧ�Ļ�ѧ����ʽ��____________________��
��3�����·�չ���ϼ������ƶ��������Ľ�����
��ʯīϩ������ͼ��������̫���ܵ�صĵ缫��������Ҫ������ʯīϩ��______________�ԡ�

�ڻ������̽����г��õ�ˮ�ࡢ�������ֲĵȡ�����ˮ��Ͳ������õ���ԭ����__________���ڸֲ������Ӹ�������Ԫ�ص�Ŀ����___________��
������ս������������SiC������Ϊ�������ϡ������½�̿��ʯӢ��Ӧ���Ƶ�SiC��ʯӢ�Ļ�ѧʽΪ________________�����·ֽ�Si(CH3)2Cl2Ҳ���Ƶ�SiC��ͬʱ������CH4��һ�ֳ����������壬д���÷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��úȼ���ŷŵ���������SO2��NOx������NaClO2��Һ��Ϊ���ռ���ͬʱ�����������������������������գ�
��1���������������������漰���Ļ�ѧ�������Ԫ���У����ڵ�������Ԫ�ص���___��д��N�ĺ�������Ų�ʽ___��
��2����֪SO2���ӵĿռ乹��Ϊ�����Σ���SO2Ϊ___��ѡ���������������Ǽ����������ӡ�
��3��������SO2��NOx������ͨ��ʢ��NaClO2��Һ�ķ�Ӧ���У���Ӧһ��ʱ�ʺ����Һ������Ũ�ȵ��й��������£��������Ӻ��Բ��ƣ���
���� | Na+ | SO42- | NO3- | OH- | Cl- |
Ũ��/��mol��L-1�� | 5.5��10-3 | 8.5��10-4 | y | 2.0��10-4 | 3.4��10-3 |
�ٷ�Ӧ����ҺpH___7������y=___mol��L-1��
��д��NaClO2��Һ����SO2�����ӷ���ʽ___��
��4�������е�SO2���ɲ��ð��������ȥ���䷴Ӧԭ��������ͼ��ʾ��
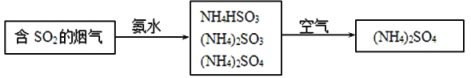
��д��SO2����ˮ��Ӧ����NH4HSO3�Ļ�ѧ����ʽ___��
��(NH4)2SO4��Һ��Ũ������������___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ԫ�أ�����A��B��CΪ����������Ԫ�أ�D��EΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
BԪ��ԭ�ӵĺ���p��������s��������1 |
Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ�I1��738 kJ��mol��1�� I2��1 451 kJ��mol��1��I3��7 733 kJ��mol��1�� I4��10 540 kJ��mol��1 |
D��ǰ�������е縺����С��Ԫ�� |
E�����ڱ��ĵ����� |
(1)��֪BA5Ϊ���ӻ����д�������ʽ_________��
(2)�Ƚ�BA2-��BA3�ļ��ǡ�ABA�Ĵ�С��BA2-____(����>������������<��)BA3�����ü۲���ӶԻ������۽��ͣ�____________��
(3)ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�ͼΪ![]() ��ͬѧ�����ĵ����Ų�ͼΥ����_______��
��ͬѧ�����ĵ����Ų�ͼΥ����_______��
(4)Eλ��_____��_____�����۵����Ų�ʽΪ______��
(5)����DԪ�صķ�����______������ԭ�ӽṹ��֪ʶ���Ͳ����������ԭ����______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������µ��ܱ������У�4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g) ��H=-905.9kJ��mol-1������������ȷ����
4NO(g)+6H2O(g) ��H=-905.9kJ��mol-1������������ȷ����
A.4molNH3��5molO2��Ӧ���ﵽƽ��ʱ�ų�����Ϊ905.9kJ
B.ƽ��ʱ��5v��(O2)=4v��(NO)
C.ƽ���ѹǿ���������ƽ��Ħ����������
D.ƽ��������¶ȣ����������NO��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NH3��һ����Ҫ�Ļ���ԭ�ϣ�����������������;�㷺��
��1����֪��
���ۼ� | ����/ kJ��mol-1 |
H�DH | 436 |
N��N | 946 |
N�DH | 391 |
ע������̬������1 molij�ֹ��ۼ���Ҫ���յ����������Ǹù��ۼ��ļ��ܡ�
N2 (g)��3 H2 (g)![]() 2 NH3 (g) H =____kJ��mol-1
2 NH3 (g) H =____kJ��mol-1
��2��һ���¶��£�����ݵ��ܱ������г���N2��H2������Ӧ��N2 ��3H2 ![]() 2NH3����ø����Ũ����ʱ��仯��ͼ1��ʾ��
2NH3����ø����Ũ����ʱ��仯��ͼ1��ʾ��
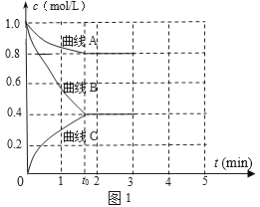
�ٱ�ʾc(N2)��������__�������A����������B��������C������
��0��t0ʱ��H2��ʾ��Ӧ����v(H2)____mol��L-1��min-1��
��������˵���÷�Ӧ�ﵽƽ�����____��
a����������ѹǿ���ٱ仯
b��2c(H2)= 3c(NH3)
c�����������������ٱ仯
d��NH3������������ٱ仯
��3��DZͧ��ʹ�õ�Һ��-Һ��ȼ�ϵ�ع���ԭ����ͼ2��ʾ��
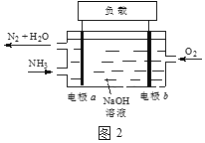
�ٵ缫b������____��
�ڵ������Һ��OH-������____�ƶ�����缫a���缫b������
�۵缫a�ĵ缫��ӦʽΪ____��
��4����ͨ��NH3��NaClO��Ӧ���Ƶû��ȼ���£�N2H4�����÷�Ӧ�Ļ�ѧ��Ӧ����ʽ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����84����Һ����1984�걱��ijҽԺ����ʹ�ö����������ճ�������ʹ�ù㷺������Ч�ɷ���NaClO��ij��ѧ�о���ѧϰС����ʵ�����Ʊ�NaClO��Һ������������̽���ͳɷֲⶨ
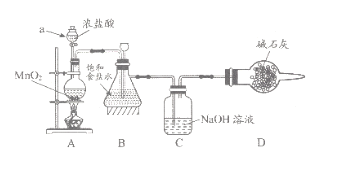
��1����ѧϰС�鰴��ͼװ�ý���ʵ��(���ּг�װ��ʡȥ)����Ӧһ��ʱ��ֱ�ȡB��Cƿ�е���Һ����ʵ�飬ʵ���������±���
��֪��1������NaClO��ҺpHΪ11��
2��25��Cʱ��������볣��Ϊ��H2CO3��K1=4.4��10��7��K2=4.7��10��11��HClO��K=3��10��8
Bƿ | Cƿ | |
ʵ��1��ȡ�����μ���ɫʯ����Һ | ��죬����ɫ | ����������ɫ |
ʵ��2���ⶨ��Һ��pH | 3 | 12 |
�ش��������⣺
������a������_____��װ��A�з�����Ӧ�����ӷ���ʽ________��
��ʵ��1��Bƿ��Һ�в��������ԭ����_________��
������Cƿ��Һ���� NaHCO3��Һ�������������������ʵ�飬Cƿ����Ϊ��ʵ��1����ɫʯ����Һ������ɫ��ʵ��2����Һ��pH=7�����ƽ���ƶ�ԭ��������ɫʯ����Һ������ɫ��ԭ��______��
��2���ⶨCƿ��Һ��NaClO����(��λ��g/L)��ʵ�鲽�����£�
��ȡCƿ��Һ20ml����ƿ�У����������ữ���������KI��Һ���ǽ�ƿ�����ڰ�����ַ�Ӧ��
����0.1000mol/LNa2S2O3����Һ�ζ���ƿ�е���Һ��ָʾ����ʾ�յ���ظ�����2~3�Σ�Na2S2O3��Һ��ƽ������Ϊ24.00ml��(��֪��I2+2S2O32��=2I��+S4O62��)
�ٲ�����Cƿ�з�����Ӧ�����ӷ���ʽΪ_______��
�ڲ����ͨ��ѡ��___��ָʾ�����ζ����յ������______��
��Cƿ��Һ��NaClO����Ϊ_____g/L(����2λС��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ķ�Ӧ·��������Ϣ��ա�

(1)A������__________��B�ļ���ʽ _________��C�Ľṹ��ʽ _____________��
(2)�ڢ١��ĸ�����Ӧ�У�����ȡ����Ӧ����_________�����ڼӳɷ�Ӧ����________��
(3)��Ӧ�۵Ļ�ѧ����ʽ��_____________________________________________________
(4)��Ӧ�ܵĻ�ѧ����ʽ��_____________________________________________________
(5)��Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ��(Cr)�Ļ������������ת����ϵ��

�����жϴ������
A.��Ӧ�ٱ���Cr2O3,�������������������
B.��Ӧ��������H2O2��������
C.��Ӧ�۷����ķ�ӦΪ2K2CrO4+H2SO4=K2Cr2O7+K2SO4+H2O
D.��Ӧ�٢ڢ��и�Ԫ�صĻ��ϼ۾������˱仯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com