
����ϩ���ױ���E����ԭ�Ϻϳɸ߷���ҩ��M���л��м���L��·�����£�

��֪��

��L����Ԫ������M�ķ���ʽ�ǣ�C15 Hl6O6��n��
�ش��������⣺
��1����2�֣�B�й����ŵ������� ��H��J�ķ�Ӧ������ ��
��2����2�֣�D�Ľṹ��ʽ�� ��
��3��(2��)F��G�Ļ�ѧ��Ӧ����ʽ ��
��4��(3�֣�K��M���ڼӾ۷�Ӧ��M�Ľṹ��ʽ�� ��
(5)(3�֣�д��K������NaOH��Һ���ȵĻ�ѧ��Ӧ����ʽ
��
��6����3�֣�д����������������C������ͬ���칹��Ľṹ��ʽ��
��
�����ڷ����廯��� ���ܷ���������Ӧ��
�ۺ˴Ź���������4��壬�ҷ����֮��Ϊ1��1��2��2��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ʵ�ó��Ľ�����ȷ���ǣ� ��
A����Һ ��ɫ�ޱ仯
��ɫ�ޱ仯 ��Һ��ΪѪ��ɫ
��Һ��ΪѪ��ɫ
���ۣ���Һ�к���Fe2+
B����Һ ��ɫ����
��ɫ���� �������ܽ�
�������ܽ�
���ۣ���Һ�к���SO42-
C��ij��Һ ƿ�ڲ�������
ƿ�ڲ������� ƿ�ڲ�������
ƿ�ڲ�������
���ۣ�����Һһ��ΪŨ����
D����ɫ��Һ ������ɫ��ζ����
������ɫ��ζ���� ʯ��ˮ�����
ʯ��ˮ�����
���ۣ�ԭ��ɫ��Һ��һ������CO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������(Al2O3) �͵����裨Si3N4���������ĸ��½ṹ�մɣ��ڹ�ҵ�����ͿƼ���������Ҫ��;��
��1��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ ��
��2������ʵ���ܱȽ�þ�����Ľ�����ǿ������ ������ţ���
a���ⶨþ�����ĵ�����ǿ��
b���ⶨ�����ʵ���Ũ�ȵ�Al2(SO4)3��MgSO4��Һ��pH
c����0.1 mol/LAlCl3��0.1 mol/L MgCl2�мӹ���NaOH��Һ
��3�����ȷ��dz��õĽ���ұ������֮һ��
��֪��4Al (s)+3O2(g) =2Al2O3(s) ��H1 = -3352 kJ/mol
Mn(s)+ O2(g) =MnO2 (s) ��H2 = -521 kJ/mol
Al��MnO2��Ӧұ������Mn���Ȼ�ѧ����ʽ�� ��
��4�������迹��ʴ������ǿ�����ױ�����ḯʴ��������������ᷴӦ�����ķ������һ����Σ��䷴Ӧ����ʽΪ ��
��5����ҵ���û�ѧ����������Ʊ������裬�䷴Ӧ���£�
3SiCl4(g) + 2N2(g) + 6H2(g)  Si3N4(s) + 12HCl(g) ��H��0
Si3N4(s) + 12HCl(g) ��H��0
ij�¶Ⱥ�ѹǿ�����£��ֱ�0.3mol SiCl4(g)��0.2mol N2(g)��0.6mol H2(g)����2L�ܱ������ڣ�����������Ӧ��5min�ﵽƽ��״̬������Si3N4(s)��������5.60g��
��H2��ƽ����Ӧ������ mol��(L��min)��
������n(SiCl4) : n(N2) : n(H2) = 3 : 2 : 6��Ͷ����ȣ���������������������ϣ�SiCl4(g)��ת����Ӧ �����������С�����䡱����
��6��298Kʱ��Ksp[Ce(OH)4]��1��10��29��Ce(OH)4���ܶȻ�����ʽΪKsp�� ��
Ϊ��ʹ��Һ��Ce4��������ȫ������������Һ�е�c(Ce4+)С��1��10��5mol��L��1�������pHΪ______���ϡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ�����߲ˡ�����Ʒ�и�����ά����C�������ԵĿ�˥�����ã����ױ�����������ij����С�����õ�ζ�����ij��֭��ά����C�ĺ������仯ѧ����ʽΪ

����˵����ȷ���� �� ��
A��������ӦΪȡ����Ӧ B���ζ�ʱ���õ�����Һ��ָʾ��
C���ζ�ʱӦ��������ƿ D������ά����C�ķ���ʽΪC6H5O6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѿ������Խ����ʪ����ʪ���ܣ�����ȣ��Ϻõ����ȡ������ԣ���Ӧ������ʮ�ֹ㷺��������ӹ��ĸ�㣬�ʵ��������ô����ɣ�����������������������߲�Ʒ���κ��ӳ������ڡ���ˮ��ɵ������Ǻ���ѿ�Ǹ�һ���ӣ�����ѿ���ǵķ���ʽΪ ��д����ѿ��������ˮ�����Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������CO2�ֱ�ͨ���CaCl2��Һ����Na2SiO3��Һ����Ca(OH)2��Һ���ܱ���Na2CO3��Һ��������Һ���а�ɫ������������
A���٢ڡ� B���ڢ� C���٢ڢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ԭ��Ӧ�У�ˮ�����ÿ���������������ԭ�����������������ǻ�ԭ�����ȷ��������ַǻ�ԭ����.���з�Ӧ�뷴ӦBr2+SO2+2H2O=H2SO4+2HBr��Ƚϣ�ˮ����������ͬ����
A��2Na2O2+2H2O =4NaOH+O2�� B��Cl2 + H2O  HClO + HCl
HClO + HCl
C��2F2+2H2O=4HF+O2 D��4Fe��OH��2+O2+2H2O=4Fe��OH��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ��Һ�д��������һ��������
A�� ��
�� ��
�� ��
�� B��
B�� ��
�� ��
�� ��
��
C�� ��
�� ��
�� ��
�� D��
D�� ��
�� ��
�� ��
��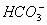
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�˵����ȷ����
A�������ĸ�ʴ�����У����ⸯʴ��������ʴ����ͬʱ����
B����ӦNH3(g)+HCl(g)��NH4Cl(s)�������¿��Է����У���÷�Ӧ�Ħ�H��0
C������Ksp(BaSO4)��Ksp(BaCO3)�����BaSO4����������ת��ΪBaCO3 ����
D��0.1 mol��L��1CH3COOH��Һ��ˮϡ�ͺ�c(OH��)��CH3COOH����Ⱦ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com