
����Ŀ����������Ԫ�أ����� A��B��C��D Ϊ����������Ԫ�أ�E��F Ϊ��������Ԫ�أ����ǵ�ԭ��������������
A Ԫ��ԭ�ӵĺ��� p �������� s �������� 3 |
B Ԫ���γɵ���������࣬���γɵ�һ�ֹ��嵥�ʹ�ҵ�ϳ������и�� |
C Ԫ�ػ�̬ԭ�� p ����� 3 ��δ�ɶԵ��� |
D ԭ�Ӻ������� p ���ȫ������� |
E �ڸ�������δ�ɶԵ�������� |
F ���γɺ�ɫ����ש��ɫ���ͺ�ɫ������������ |
��������������Ϣ���ش����⣺
��1��A ��±�����ڹ�ҵ������Ҫ���ã�A ������±����ķе����±���ʾ��
±���� | AF3 | ACl3 | ABr3 | AI3 |
�е�/K | 172 | 285 | 364 | 483 |
������±����е��������ߵ�ԭ����_________________��
�� ACl3��LiAH4 ��A ԭ�ӵ��ӻ������������Ϊ______��_______���� A3N3H6 ��Ϊ�ȵ�����ķ��ӵĽṹ��ʽΪ___________��
����AF3���ӽṹ���ͷ�Ӧ AF3(g)+NH4F(s)=NH4AF4(s)�ܹ�������ԭ��_________________��
��2��ijͬѧ����������Ϣ���ƶ�
��B��̬ԭ�ӵĺ��������Ų�Ϊ ����ͬѧ�����ĵ����Ų�ͼΥ����________��
����ͬѧ�����ĵ����Ų�ͼΥ����________��
�� ��֪Ԫ�� B ��һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����________��
A�������к��з��Ӽ���� B�����ں��м��Լ��ķǼ��Է���
C��ֻ����4��sp-s�ĦҼ���1��p-p�Ħм� D�����⻯������� B ԭ�Ӳ��� sp2 �ӻ�
��3��D ��̬ԭ����������ߵĵ��ӣ���������ڿռ���______������ԭ�ӹ����______�Ρ�
��4��д�� E ԭ�ӵĵ����Ų�ʽ______________��
��5����д�� F Ԫ����Χ�����Ų�ʽ_________���� FSO4��Һ�еμ���CԪ���⻯���ˮ��Һ����������ɫ��������������ܽ⣬�õ�����ɫ����Һ����д�������ܽ�����ӷ���ʽ__________��
���𰸡��ṹ���ƣ������������Ӽ������������۷е��������� sp2�� sp3 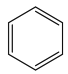 AF3 ��BF3 ��B���пչ������NH4F�е�F�йµ��Ӷԣ������ܹ���Ӧ ���ع��� BD 3 ���� 1s22s22p63s23p63d54s1 3d104s1 Cu(OH)2 +4NH3 = [Cu(NH3)4]2++2OH-
AF3 ��BF3 ��B���пչ������NH4F�е�F�йµ��Ӷԣ������ܹ���Ӧ ���ع��� BD 3 ���� 1s22s22p63s23p63d54s1 3d104s1 Cu(OH)2 +4NH3 = [Cu(NH3)4]2++2OH-
��������
��1��A��B��C��D Ϊ����������Ԫ�أ�E��F Ϊ��������Ԫ����A Ԫ��ԭ�ӵĺ��� p �������� s �������� 3�ĵ����Ų�Ϊ1S22S22P1֪AΪ��B��; B Ԫ���γɵ���������࣬���γɵ�һ�ֹ��嵥�ʹ�ҵ�ϳ������и��֪BΪ̼��C������C Ԫ�ػ�̬ԭ�� p ����� 3 ��δ�ɶԵ���֪����N����D ԭ�Ӻ������� p ���ȫ���������P����E �ڸ�������δ�ɶԵ����������Ϊ����Cr����F ���γɺ�ɫ����ש��ɫΪCuO���ͺ�ɫ(CuO)������������,����ΪCu;
����ΪAF3ΪBF3 BCl3 BBr3 BI3����±�����ǽṹ���ƣ��������������Ӽ������������۷е��������ߡ��𰸣��ṹ���ƣ������������Ӽ������������۷е������������� ACl3ΪBF3����ƽ�������Σ��ӻ���ʽΪsp2��LiAH4ΪLiBH4 ��B ԭ��Ϊsp3�����ӻ������������Ϊsp2 sp3���ȵ�����ָԭ��������ͬ���۵���������ͬ�ķ��ӣ��ȵ�����������ƵĻ�ѧ�������������� A3N3H6 ��Ϊ�ķ���ʽΪC6H6,�ṹ��ʽΪ![]() ��
��
��AF3�ķ���ʽΪBF3�� BF3 ��B���пչ������NH4F�е�F�йµ��Ӷԣ������ܹ���Ӧ��AF3(g)+NH4F(s)=NH4AF4(s)���𰸣�AF3 ��BF3 ��B���пչ������NH4F�е�F�йµ��Ӷԣ������ܹ���Ӧ��
��2�������ع���Ҫ������������ȵ���ռ��һ���������������״̬��ͬ��BΪ̼�����̬ԭ�ӵĺ��������Ų�ӦΪ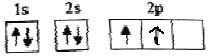 ������ͬѧ�����ĵ����Ų�ͼΥ���˺��ع��𰸣� ���ع�����
������ͬѧ�����ĵ����Ų�ͼΥ���˺��ع��𰸣� ���ع�����
��Ԫ��BΪ̼��һ���⻯������Ҫ�Ļ���ԭ��,���Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־,Ϊ��ϩ,�ṹʽΪ![]() ,����֪�������к���
,����֪�������к���![]() �Ǽ��Լ���
�Ǽ��Լ���![]() ���Լ�,Ϊƽ���η���,����5��
���Լ�,Ϊƽ���η���,����5��![]() ����1��
����1��![]() ��,ÿ��C�γ�3��
��,ÿ��C�γ�3��![]() ��,Ϊ
��,Ϊ![]() �ӻ�,��ˣ�������ȷ����:BD;
�ӻ�,��ˣ�������ȷ����:BD;
��3��DΪP���������Ų�Ϊ1S22S22P63S23P3, ��̬ԭ����������ߵĵ��ӣ���������ڿռ���3������ԭ�ӹ���������Ρ�
��4�� EΪ��Ϊ24��Ԫ�أ�����ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p63d54s1��
��5�� F Ԫ��ΪCu,������Ų�ʽΪ1s22s22p63s23p63d104s1������Χ�����Ų�ʽ3d104s1 ��FSO4ΪCuSO4��Һ���� CuSO4��Һ�еμ���CԪ���⻯��ΪNH3,��ˮ��ҺΪ��ˮ����������ɫ����Cu(OH02����������ܽ⣬�õ�����ɫ����ҺΪ[Cu(NH3)4]2+���˳����ܽ�����ӷ���ʽCu(OH)2 +4NH3 = [Cu(NH3)4]2++2OH-��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�����ײ������ǵ�����Ͽ�ѧ�о���ǰ�أ����о��ɹ��㷺Ӧ���ڴ��������¿�ѧ�С���ν�����ײ�������ָ�о�������������ֱ���Ӽ�������ʮ���IJ��ϣ��罫���ײ��Ϸ�ɢ����ɢ���У����û������ܾ��е�������________��
a����ȫ������ֽ
b���ж����ЧӦ
c������Һ��ʽ�״
d����������һ��������Һ
(2)�ѵ�����Һ���ڷ�ˮ�У��Ƴɵ��۽��壬������Һ�͵��۽���������õķ�����_______________��
��.�����ЧӦ�����ֽ�������Һ��һ����õķ�����
(1)��ͼ��ʾ����ʵ�����н���Fe(OH)3���嶡���ЧӦʵ���ʾ��ͼ����ͼ����һ�����ԵĴ�����______��ԭ����____________(�Դӷ�ɢϵ�ķ������˵��)��
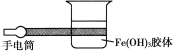
(2)���������й۲춡���ЧӦ������Ϊһ�������п��ܹ۲쵽�������ʱ����________��������_________________��
(3)ȥ���ֹ۲춡���ЧӦ�����㣬������������������ڼ���������ЧӦ�ķ����������һ������Ϊ�������ķ�����__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��ͬѧ�������������������Բ��˲�����֮˵������Ȥ�����ʵ��������̽�����������ױ����������е���Ԫ���Բ�����������ʽ���ڣ�����������һ�ֵ���ɫ������ˮ�ľ��塣
I.��������Ԫ�غ����IJⶨ
(1)ȡ100gϴ�����ɵ����ʲ��ˣ������װ��___________�У���������ճɻҽ���
(2)���ҽ���25mL 2mol/L������Һ�ܽ⣬�������һ���ӣ����˺�μ�����H2O2��Һ��ϡ����100mL��ȡ2mL����5��KSCN��Һ��H2O2�������ɹ�������Һ���ȱ�����ɫ����ɫ��ԭ����_________________________________��
(3)ȡ��ͬŨ�ȵ�___________[����(NH4)2Fe(SO4)2������NH4Fe(SO4)2��]����Һ��2mL���ֱ�μ�5��KSCN��Һ�������벽��(2)����Һ��ɫ��ӽ��ı���ҺŨ��Ϊ0.4��10-3mol/L��
(4)������ɵã�ÿ100g���ʲ����к�����Ԫ�ص�����ԼΪ___________ mg(����1λ��Ч����)��������5%�����ʣ�Ϊ����ÿ��20mg����Ԫ������ÿ����Ҫ�Բ���___________kg��
��.������������(FeC2O4��nH2O)�ķֽ�ʵ��
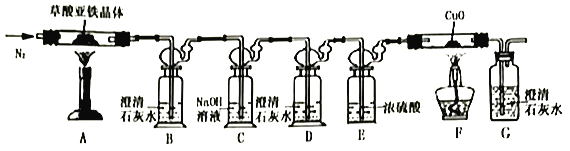
(1)�ӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ����___________��
(2)ʵ�鿪ʼ��װ��B�г��ֻ���֤���ֽ�����д���CO��������___________������ַ�Ӧ��Ĺ��������Ͷ�뾭��е�ϡ�����У�������ȫ�ܽ���������ų���ȡ��ӦҺ����KSCN��Һ��Ѫ��ɫ��
(3)��ȡ7.2g���������������װ��AӲ�ʲ������У����Ⱥ�(��������跴Ӧ��ȫ)�����װ��AӲ�ʲ������в������2.88g��װ��FӲ�ʲ������й�����������0.64g���������������ֽ�Ļ�ѧ����ʽΪ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijԪ��ԭ�ӵ�ԭ�Ӻ������������Ӳ㣬������������4����ԭ�Ӻ��ڵ��������ǣ� ��
A. 14B. 15C. 16D. 17
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С�����Fe(OH)3������Ʊ�ʵ�鲢������������ʡ�
��1����������FeCl3��Һ�ֱ��������������,���γɽ������______________(�����)��
A.��ˮ B.��ˮ C.NaOHŨ��Һ D.NaClŨ��Һ
��2��д���Ʊ�Fe(OH)3����Ļ�ѧ��Ӧ����ʽ:________________��
��3������֤���Ƶõ������ǽ���?�������������:__________________��
��4��ȡ�����ƵõĽ�������Թ���,�ټ�������(NH4)2SO4��Һ,�۲쵽��������__________, ���������Ϊ�����________________��
��5��Fe(OH)3�������ȶ����ڵ���Ҫԭ����________(�����)��
A. ����ֱ��С��1nm B. �����������
C. �����������˶� D . ����������ֽ
��6��Fe(OH)3����������FeCl3��Һ��ʵ�������________(�����)��
A. Fe(OH)3�������ӵ�ֱ����1~100nm֮��
B. Fe(OH)3������ж����ЧӦ
C. Fe(OH)3�����Ǿ�һ�ķ�ɢϵ
D. Fe(OH)3����ķ�ɢ������������ֽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ������װ�ý����й�Cl2��ʵ�顣����˵������ȷ����


A. ��ͼ�У�ʵ������֤��������Ư�����ã���ˮ��Ư������
B. ��ͼ�У���Cl2����ζ
C. ��ͼ�У������ػ�ɫ����
D. ��ͼ�У�������ɱ�֤��Cl2����NaOH��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ��ֻ���ܺ�������K+��NH4+��Fe2+��Al3+��Cl-��SO42-��CO32-��AlO2-�е����������ӣ�����Ũ�Ⱦ�Ϊ0.1mol��L-1��ijͬѧ����������ʵ�飺
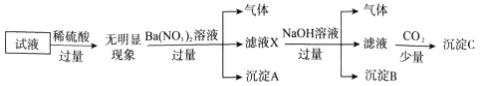
����˵����ȷ���ǣ� ��
A. ��ȷ��ԭ��Һ���Ƿ���Al3+��Cl-
B. ��ҺX�д������ڵ���������NH4+��Fe2+��Ba2+
C. ��ȷ������C�ijɷ�
D. ԭ��Һ�д��ڵ�����ΪNH4+��Fe2+��Cl-��SO42-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ��������ֱ���Na2CO3��AgNO3��BaCl2������������ɫ��Һ�е�һ�֣�����������Ӧ����������£��ף���![]() �������ף���
�������ף���![]() �������ң���
�������ң���![]() ������������
������������![]() �������ң���
�������ң���![]() ��ɫ��ζ���塣��ס��ҡ�������������Һ������(����)
��ɫ��ζ���塣��ס��ҡ�������������Һ������(����)
A. BaCl2��Na2CO3��AgNO3������
B. BaCl2��Na2CO3�����ᡡAgNO3
C. Na2CO3�����ᡡAgNO3��BaCl2
D. AgNO3�����ᡡBaCl2��Na2CO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҩ��H��һ�ֺϳ�·�����£�

��֪��R-C![]() CH+H2O
CH+H2O![]()
![]()
��ش���������
(1)A��������___________��B��C�ķ�Ӧ�������Լ���______________________��
(2)E�к���������������___________��G��H�ķ�Ӧ������___________��
(3)д��E��F��Ӧ�Ļ�ѧ����ʽ_________________________________��
(4)1���л���C���������___________��ԭ�ӹ�ƽ�档
(5)������F�ж���ͬ���칹�壬ͬʱ��������������ͬ���칹����___________�֣����к˴Ź���������5����ҷ����֮��Ϊ1�U2�U2�U2�U3�����ʵĽṹ��ʽΪ___________��
���ܷ���ˮ�ⷴӦ�������ڷ��㻯�������ܷ���������Ӧ
(6)����������Ϣ���Ա�ȩ(CH2CH2CHO)Ϊ��Ҫԭ�Ϻϳ�![]() �������Լ���ѡ)����ƺϳ�·�ߣ�______��
�������Լ���ѡ)����ƺϳ�·�ߣ�______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com