
(7��)���к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȡ�(��ƿ��װ����Һ)
���ƴ���Һ����2.0g������������(���ʲ������ᷴӦ)�Ĺ����ռ���Ʒ����200mL��Һ��
(1) �ζ���ʢװ0.20mol/L�����ҺӦ������ʽ�ζ��ܣ��ζ�ʱ������ʢ����Һ����ƿ�мӷ�̪��Ϊָʾ�����ζ�����������Ӧ��ע��__________________________________��
�ζ��յ��������:___________________________________
(2) �й����ݼ�¼���£�
| �ζ���� | ����Һ���(ml) | �����������Һ�����(ml) | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.50 | 20.55 |
| 2 | 20.00 | 6.00 | 25.95 |
���ȼ��㣺�ռ���Ʒ�Ĵ���Ϊ________________��
(3) �Լ��ּٶ���������ۣ�(����Ӱ�졢ƫ�ߡ�ƫ��)��˵�����ⶨ�Ľ��ָ�ռ���Ʒ�Ĵ��ȣ�
����������ˮ��ϴ��ƿ�����ʹ�ⶨ�Ľ��___________________
�����ζ�ǰ������ˮ��ϴ��ʽ�ζ��ܺ�װ�����ᣬ���ʹ�ⶨ���__________
�۵μ������ٶȹ��죬δ������տ�����Һ��ɫ������ֹͣ�ζ������ʹ�ⶨ���__________��
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����������һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
(7��)���к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȡ�(��ƿ��װ����Һ)
���ƴ���Һ����2.0g������������(���ʲ������ᷴӦ)�Ĺ����ռ���Ʒ����200mL��Һ��
(1) �ζ���ʢװ0.20mol/L�����ҺӦ������ʽ�ζ��ܣ��ζ�ʱ������ʢ����Һ����ƿ�мӷ�̪��Ϊָʾ�����ζ�����������Ӧ��ע��__________________________________��
�ζ��յ��������:________________________________
(2) �й����ݼ�¼���£�
| �ζ���� | ����Һ���(ml) | �����������Һ�����(ml) | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.50 | 20.55 |
| 2 | 20.00 | 6.00 | 25.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ����һ�и���5��ģ�⿼�������ۺϻ�ѧ�Ծ����������� ���ͣ������
(15��)�������ᣨH2NSO3H����һԪ����ǿ�ᣬ����ˮ��Һ�����������Ҵ����ڹ�ҵ������������ϴ������ȼ�����ǻ����ȡ�������ƷΪ��ɫ��ĩ���ڳ����£�ֻҪ���ָ��ﲻ��ˮ�Ӵ�������İ��������ʪ���Ƚ��ȶ��������в��ӷ�����ζ�Ͷ����嶾�Լ�С���ص㡣ijʵ���������غͷ������ᣨ����SO3�����ᣩΪԭ�Ϻϳɰ��������·������ ���ǻ����������������ķ�ӦΪ��
��CO(NH2)2(s) �� SO3(g)  H2NCONHSO3H(s) ��H��0
H2NCONHSO3H(s) ��H��0
��H2NCONHSO3H + H2SO4 2H2NSO3H + CO2��
2H2NSO3H + CO2��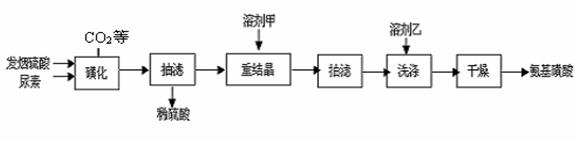
��1����ͼ�ǡ��ǻ������̵�ʵ��װ�ã���ѹ��Һ©���������� ____________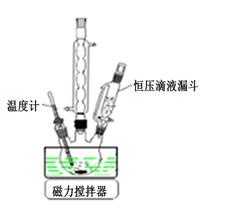
��2������ʱ�����þ���Ҫ���ܼ��Ҵ�ϴ�ӣ���ϴ�ӵľ��������
��3��ʵ����̵����۷�����
���ؽᾧʱ���ܼ��ף�10%��12%�����ᣩ���ؽᾧ���ܼ��ö�����ˮ���ܼ���ԭ����
�� ���ǻ��������¶�����ʵĹ�ϵ��ͼ��1�������Ʒ�Ӧ�¶�Ϊ75~80��Ϊ�ˣ����¶ȸ���80�棬��������IJ��ʻή�ͣ�ԭ���� ��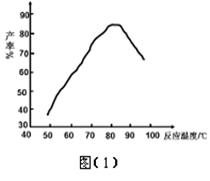
��4���ⶨ��Ʒ�а������ᴿ�ȵķ������£���ȡ7.920g��Ʒ���l000mL����Һ����ȡ25.00mL����Һ����ƿ�У�����2mL 0.2000mol��L��1ϡ���ᣬ�õ��۵⻯���Լ���ָʾ������μ���0.08000mol��L��1NaNO2��Һ������Һǡ�ñ���ʱ������NaNO2��Һ25.00mL����ʱ��������ǡ�ñ���ȫ������N2��NaNO2�Ļ�ԭ����ҲΪN2��
���Է�̪Ϊָʾ������NaOH��������к͵ζ�Ҳ�ܲⶨ��Ʒ�а�������Ĵ��ȣ��ⶨ���ͨ����NaNO2��ƫ�ߣ�ԭ���ǰ��������л��� _____ ���ʡ�
��д��NaNO2�ζ����еĻ�ѧ����ʽΪ�� ��
�������Ʒ�а����������������__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����5��ģ�⿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
(15��)�������ᣨH2NSO3H����һԪ����ǿ�ᣬ����ˮ��Һ�����������Ҵ����ڹ�ҵ������������ϴ������ȼ�����ǻ����ȡ�������ƷΪ��ɫ��ĩ���ڳ����£�ֻҪ���ָ��ﲻ��ˮ�Ӵ�������İ��������ʪ���Ƚ��ȶ��������в��ӷ�����ζ�Ͷ����嶾�Լ�С���ص㡣ijʵ���������غͷ������ᣨ����SO3�����ᣩΪԭ�Ϻϳɰ��������·������ ���ǻ����������������ķ�ӦΪ��
��CO(NH2)2(s) �� SO3(g)  H2NCONHSO3H(s)
��H��0
H2NCONHSO3H(s)
��H��0
��H2NCONHSO3H + H2SO4  2H2NSO3H + CO2��
2H2NSO3H + CO2��
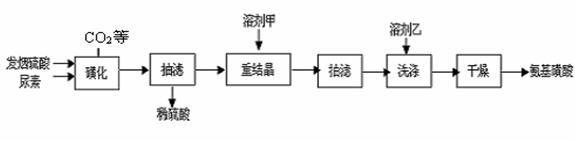
��1����ͼ�ǡ��ǻ������̵�ʵ��װ�ã���ѹ��Һ©���������� ____________
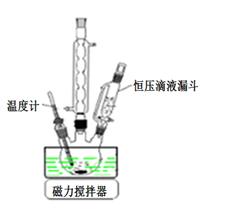
��2������ʱ�����þ���Ҫ���ܼ��Ҵ�ϴ�ӣ���ϴ�ӵľ��������
��3��ʵ����̵����۷�����
���ؽᾧʱ���ܼ��ף�10%��12%�����ᣩ���ؽᾧ���ܼ��ö�����ˮ���ܼ���ԭ����
�� ���ǻ��������¶�����ʵĹ�ϵ��ͼ��1�������Ʒ�Ӧ�¶�Ϊ75~80��Ϊ�ˣ����¶ȸ���80�棬��������IJ��ʻή�ͣ�ԭ���� ��
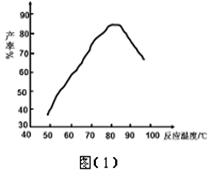
��4���ⶨ��Ʒ�а������ᴿ�ȵķ������£���ȡ7.920g ��Ʒ���l000mL����Һ����ȡ25.00mL����Һ����ƿ�У�����2mL 0.2000mol��L��1ϡ���ᣬ�õ��۵⻯���Լ���ָʾ������μ���0.08000mol��L��1NaNO2��Һ������Һǡ�ñ���ʱ������NaNO2��Һ25.00mL����ʱ��������ǡ�ñ���ȫ������N2��NaNO2�Ļ�ԭ����ҲΪN2��
���Է�̪Ϊָʾ������NaOH��������к͵ζ�Ҳ�ܲⶨ��Ʒ�а�������Ĵ��ȣ��ⶨ���ͨ����NaNO2��ƫ�ߣ�ԭ���ǰ��������л��� _____ ���ʡ�
��д��NaNO2�ζ����еĻ�ѧ����ʽΪ�� ��
�������Ʒ�а����������������__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
(7��)���к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȡ�(��ƿ��װ����Һ)
���ƴ���Һ����2.0g������������(���ʲ������ᷴӦ)�Ĺ����ռ���Ʒ����200mL��Һ��
(1) �ζ���ʢװ0.20mol/L�����ҺӦ������ʽ�ζ��ܣ��ζ�ʱ������ʢ����Һ����ƿ�мӷ�̪��Ϊָʾ�����ζ�����������Ӧ��ע��__________________________________��
�ζ��յ��������:________________ ___________________
(2) �й����ݼ�¼���£�
|
���� |
����Һ���(ml) |
�����������Һ�����(ml) |
|
|
�ζ�ǰ |
��� |
||
|
1 |
20.00 |
0.50 |
20.55 |
|
2 |
20.00 |
6.00 |
25.95 |
���ȼ��㣺�ռ���Ʒ�Ĵ���Ϊ________________��
(3) �Լ��ּٶ���������ۣ�(����Ӱ�졢ƫ�ߡ�ƫ��)��˵�����ⶨ�Ľ��ָ�ռ���Ʒ�Ĵ��ȣ�
����������ˮ��ϴ��ƿ�����ʹ�ⶨ�Ľ��___________________
�����ζ�ǰ������ˮ��ϴ��ʽ�ζ��ܺ�װ�����ᣬ���ʹ�ⶨ���__________
�۵μ������ٶȹ��죬δ������տ�����Һ��ɫ������ֹͣ�ζ������ʹ�ⶨ���__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com