
�״���һ�ֳ��õ�ȼ�ϣ���ҵ�Ͽ�����CO��H2��һ�������ºϳɼ״���
��1����֪CO��g����H2��g����CH3OH��1����ȼ���ȡ�H�ֱ�Ϊ��-283.0kJ��mol��-285.8 kJ/mol��-726.5kJ/mol����CO�ϳɼ״����Ȼ�ѧ����ʽΪ�� ��
��2���ں����ܱ�������CO��H2������Ӧ���ɼ״���������Ũ���ڲ�ͬ�����µı仯״����ͼ��ʾ����ʼʱ������Ũ�����ߺ�8���Ӻ�״���Ũ������δ������4���Ӻ�8���Ӹı��������ͬ���� 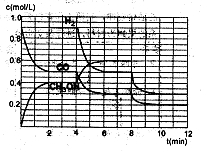
������˵����ȷ����
| A����ʼʱn��H2��Ϊ1��7mol |
| B����������ѹǿ�㶨ʱ��˵����Ӧ�ﵽƽ��״̬ |
| C��4����ʱ���ı�������������¶� |
| D��7����ʱ��v��CO��=v��CH3OH�� |
��ѧ��Ӧʽ��ʽδ��ƽ�ľ���1��
��1��CO(g)��2H2(g)��CH3OH(l) ��H����128.1kJ/mol ��2�֣�����ʽ1�֣���Ӧ��2�֣�״̬�����1�֣����������÷֡���
��2�� �� BD ��2�֣���ѡ��1�֣��д�ѡ���÷֣�
�� 0.4mol/(L��min) ��6.67��10��3 mol��L��1��s��1��2�֣���λ���÷֣�
�� 0.99 ��2�֣���ֵ��1.0��0.98֮����÷֣�д�ɡ�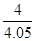 ����
���� �� ��1�֣���λ����Ҫ��
�� ��1�֣���λ����Ҫ��
��
��3���� CH3OH��6e����H2O��CO2����6 H�� ��2�֣��ޡ������������������۷֣�������д���÷֡���
�� ����Ʒ��1�֣�д��Al�����������ɣ�������д���÷֣�
2Al��6e����6HCO3����Al2O3��6CO2����3H2O��3�֣���Al��3e����Al3����Al3����3HCO3����Al(OH)3����3CO2����2Al(OH)3��Al2O3��3H2O����1�֣��ޡ������������������۷֣�������д���÷֡���
���������������1����д���������ʵ�ȼ���ȵ��Ȼ�ѧ����ʽ��
�� CO��g��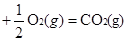 ��H1����283.0kJ��mol
��H1����283.0kJ��mol
�� H2��g��+  ��H2����285.8 kJ/mol
��H2����285.8 kJ/mol
�� CH3OH��1��+  ��H3����726.5kJ/mol
��H3����726.5kJ/mol
��д��Ŀ�귽��ʽ��CO(g)��2H2(g)��CH3OH(l) ��H4
���ݸ�˹���ɣ� ��+�ڡ�2-�ۼ��õ�Ŀ�귽��ʽ�� ��H4= ��H1+��H2��2-��H3=��128.1kJ/mol
����ͼ�� CO(g)��2H2(g)��CH3OH(l)
��ʼ����mol/L�� 0.9 x 0
0-2min��mol/L�� ��0.4 ��0.8 0.4
2-4min��mol/L����ƽ�⣩ 0.5 0.9 0.4
4-6min��mol/L�� ��0.2 ��0.4 0.2
6-8min��mol/L����ƽ�⣩ 0.3 0.5 0.6
8-10min��mol/L�� ��0.1 ��0.2 0.1
10-12min��mol/L����ƽ�⣩0.2 0.3 y
��A���ʼʱŨ��x=C��H2��Ϊ1��7mol/L���������δ֪���������ʵ���Ҳδ֪��A�����
B���������һ�����ݵ���������Ӧ��һ���ǵ������Ӧ����˵�����������ʵ������ٸı䣬��ѹǿ���ٸı䣬��Ӧ���ﵽƽ��״̬����ȷ��
C�4minʱ��ƽ�������ƣ����Ҹ����ʵ�Ũ�����仯������Ӧ���ǽ��µ������£�C�����
D�7minʱ����Ӧ�ٴδﵽƽ�⣬��ʱv��CO��=v��CH3OH����D����ȷ��
��0~2min��ƽ����Ӧ����v��H2��=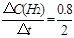 ="0.4" mol��L-1��min-1
="0.4" mol��L-1��min-1
����3minʱ�÷�Ӧ��ƽ�ⳣ��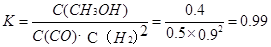
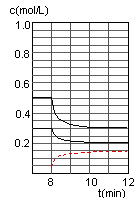
���ڵ�8minʱ��CO��Ũ�ȼ���0.1mol��L-1��H2��Ũ�ȼ���0.2mol��L-1����֪ƽ���������ƶ���CO��H2��Ũ�ȶ�����С������ʱ���������ϴ�ƽ���ƶ�����������ͬ������ֻ���Ǽ��ټ״���Ũ�ȣ�����ʹƽ�������ƶ�������ƽ���ƶ���˲�䣬�״���Ũ���Ǻ��ٵģ�Ӧ��Ҫ����0.05mol��L-1��Ȼ��������0.1mol��L-1���ڵ�10minƽ�⣬����Ũ��y������0.15mol��L-1��
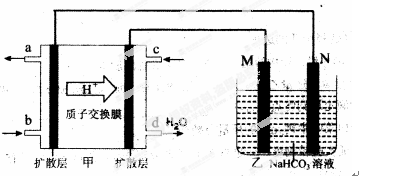
��3����װ���м���һ��ȼ�ϵ��װ�ã����ұߵ�װ�����ṩ���ܣ���װ����һ������װ�á�
�ټ������������ұߣ��Ӷ���֪��װ�����Ǹ�������������������װ����M��������N�����������е�صĸ�����ӦʽΪ��CH3OH��6e����H2O��CO2����6 H�� Ҫע��������Һ�����ڽ��д��ݡ�
���ҳ���һ����Ʒ���桰�ۻ���װ�ã������ֱ�Ϊ����Ʒ��ʯī��������M����������Ʒ������N������ʯī����������ۻ������������Al2O3��ͬʱ������Һ������ΪNaHCO3����缫��ӦΪ��2Al��6e����6HCO3��=Al2O3��6CO2����3H2O
���㣺 �����Ȼ�ѧ����ʽ����д����ѧƽ��ԭ������ͼ�����⡢�绯ѧ�ۺϡ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�á�������������������գ�
��1��ͬ��ͬѹ�£�H2(g)+Cl2(g)��2HCl(g)�����պ͵�ȼ�����Ħ�H����ѧ��������ͬ���ֱ�Ϊ��H1����H2����H1______��H2��
��2����ͬ�����£�2mol��ԭ�������е����� 1mol����������е�������
��3����֪����ʱ���ױȰ����ȶ����Ƚ����з�Ӧ�Ц�H�Ĵ�С����H1_____��H2��
��4P(���ף�s) +5O2(g)��2P2O5(s) ��H1����4P(���ף�s)+5O2(g)��2P2O5(s) ��H2��
��4����֪��101 kPaʱ��2C(s) +O2(g)��2CO(g) ��H����221kJ��mol��1����̼��ȼ������ֵ 110.5 kJ��mol��1��
��5����֪��ϡ��Һ�У�H��(aq)+OH�� (aq)��H2O(l) ��H����57.3kJ/mol����Ũ������ϡNaOH��Һ��Ӧ����1 molˮ���ų������� 57.3 kJ��
��6�����淴Ӧ��aA(��)+bB(��) cC(��)+dD(��)����H��Q������ͼ�ش�
cC(��)+dD(��)����H��Q������ͼ�ش�
P1 ______ P2���ڣ�a+b��______��c+d������t1��______ t2�档
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����仯�����ڹ�ũҵ������������������Ҫ���á���ش��������⣺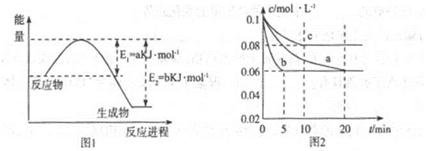
��1��ͼ1��1molNO2��1molCO��Ӧ����CO2��NO���������DZ仯ʾ��ͼ(a��b������0��)��֪:2CO(g)+2NO(g)=N2(g)+2CO2(g)��H=-ckJ��mol-1��c>0��
��д��CO��NO2��ԭ��N2ʱ���Ȼ�ѧ����ʽ____________��
��2��ͼ2��ʵ������������ͬ�������ܱ������кϳɰ�ʱ��N2��Ũ����ʱ��ı仯����(��a��b��c��ʾ������֪������������ʼ����Ũ�Ⱦ�Ϊ��c(N2)=0.1mol��L-1��c(H2)=0.3mol��L-1���ϳɰ��ķ�Ӧ��N2(g)+3H2(g) 2NH3(g)��H<0
2NH3(g)��H<0
�ټ�����a��ƽ��ʱH2��ת����Ϊ______��
����ͼ2��֪��b��c����һ��������a��ͬ����c�������ı������______��
��д���ж�b��a������ͬ������____________��
��3������ͼ2��c�����ºϳɰ����ݻ��̶�������֪��ѧƽ�ⳣ��K���¶�(T)�Ĺ�ϵ���±�:
����ȷ��K1����Դ�С��K1______4.1x106(��д��>����-����<����
�����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�����ݵ� ��______(�������ĸ����
A��������NH3��Ũ�ȱ��ֲ��� B��2v(N2)(����=v(H2)(�棩
C��������ѹǿ���ֲ��� D�����������ܶȱ��ֲ���
��4����NH4Cl��Һ�����Ե�ԭ����(�����ӷ�Ӧ����ʽ��ʾ )______��
��250Cʱ����pH=x��ˮ��pH=y������(��x+y=14,x>11)�������Ϻ�������Һ�и������ӵ�Ũ�ȹ�ϵ��ȷ����
A��[SO42-]>[NH4+]>[H+]>[OH-]
B��[NH4+]>[SO42-]>[OH-]>[H+]
C��[NH4+]+[H+]>[OH-]+[SO42-]
D��[NH4+]>[SO42-]>[H+]>[OH-]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
SNCR��SCR��һ�����͵�������������(��ȥ�����е�NOx�������������£� 
��1����Ӧ2NO��2CO 2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������
2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������
��2��SNCR��SCR�����з�������Ҫ��Ӧ�У�
4NO(g)��4NH3(g)��O2(g) 4N2(g)��6H2O(g) ��H����1627.2kJ?mol��1��
4N2(g)��6H2O(g) ��H����1627.2kJ?mol��1��
6NO(g)��4NH3(g) 5N2(g)��6H2O(g) ��H����1807.0 kJ?mol��1��
5N2(g)��6H2O(g) ��H����1807.0 kJ?mol��1��
6NO2(g)��8NH3(g) 7N2(g)��12H2O(g) ��H����2659.9 kJ?mol��1��
7N2(g)��12H2O(g) ��H����2659.9 kJ?mol��1��
��ӦN2(g)��O2(g) 2NO(g)�Ħ�H�� kJ?mol��1��
2NO(g)�Ħ�H�� kJ?mol��1��
��3��NO��NH3��Ag2O��������ķ�Ӧ�������¶ȵı仯����ͼ��
����ͼ���Կ�����������������Ӧ�� ������������������������½��С�
�����ŷ�Ӧ�¶ȵĽ�һ�����ߣ���������������NO��ת���������½��Ŀ���ԭ���� ��
��4��NO2Ҳ��������[CO(NH2)2]��ԭ��д��������NO2��Ӧ�Ļ�ѧ����ʽ�� ��
��5��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ����ͼ11���õ����ʹ�ù�����ʯīI�缫������������Y����缫��ӦΪ ��������1molY������������Ҫ���ı�״�������������Ϊ L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о����仯�������������Ҫ���塣
��1��Cu2S�ڸ��������·������·�Ӧ��
2Cu2S(s)+3O2(g)=2Cu2O(s)+2SO2(g) �SH=��773kJ/mol
���÷�Ӧ��1.2mol����ת��ʱ,��Ӧ�ͷų�������Ϊ kJ��
��2�����Ṥҵ�������漰��Ӧ��2SO2(g)+O2(g) 2SO3(g)��SO2��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
2SO3(g)��SO2��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��ѹǿ��P1 P2�����������=����<������
��ƽ�ⳣ����A�� B�㣨���������=����<������
��200���£���һ������SO2��O2�������������ܱ������У���10min���������и����ʵ����ʵ���Ũ�����±���ʾ:
| ���� | SO2 | O2 | SO3 |
| Ũ�ȣ�mol/L�� | 0.4 | 1.2 | 1.6 |
 ������֪���¶���H2SO3�ĵ��볣����Ka1=1.0��10-2 mol/L��Ka2=6.0��10-3 mol/L��
������֪���¶���H2SO3�ĵ��볣����Ka1=1.0��10-2 mol/L��Ka2=6.0��10-3 mol/L���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��2H2(g)+O2(g)=2H2O(l) ��H=��572kJ/mol ��ش��������⣺
��1�������������ܺ���������������ڡ�����С�ڡ����ڡ�����Ӧ�������ܺ�
��2����2 mol������ȫȼ������ˮ��������ų������� 572 kJ���������������������
����֪1molCu(s)������O2(g)������Ӧ������CuO(s)���ų�157kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣���ѧ����Դ����������������ʮ����Ҫ�����á�
��1���̲��ں��Ŀ�ȼ���Ǹ�ѹ���γɵ��������ļ���ˮ������壮����֮Ϊ��δ����Դ������25�桢101 kPa�£�1g������ȫȼ�����ɺ�Һ̬ˮʱ����55.6 kJ������ȼ�յ��Ȼ�ѧ����ʽΪ ______����ͬ�����£�356 g��ȼ��������ʽΪCH4��9H2O��Mr��178���ͷŵļ���������ȫȼ������CO2��Һ̬ˮ���ų�������Ϊ_______kJ��
��2��������(CH3OCH3)����ɫ���壬����Ϊһ��������Դ��������ࡢ��Ч���������ܡ��ɺϳ���(���ΪH2��CO��������CO2)ֱ���Ƹ������ѣ����е���Ҫ���̰��������ĸ���Ӧ��
�״��ϳɷ�Ӧ��
����CO��g��+2H2��g���TCH3OH��g�� ��H1����90.1kJ?mol-1
����CO2��g��+3H2��g���TCH3OH��g��+H2O��g�� ��H2����49.0kJ?mol-1
ˮú���任��Ӧ������CO��g��+H2O��g���TCO2��g��+H2 ��g�� ��H3����41.1kJ?mol-1
�����Ѻϳɷ�Ӧ��������2CH3OH��g���TCH3OCH3��g��+H2O��g����H4����24.5kJ?mol-1
�ٷ��������Ѻϳɷ�Ӧ(iv)����COת���ʵ�Ӱ��___________________________________��
����H2��COֱ���Ʊ������ѣ���һ����Ϊˮ���������Ȼ�ѧ����ʽΪ��__________________�����ݻ�ѧ��Ӧԭ������������ѹǿ��ֱ���Ʊ������ѷ�Ӧ��Ӱ��_________________________________��
��3��������ֱ��ȼ�ϵ�ؾ��������졢Ч�ʸߵ��ŵ㡣�������Ϊ���ԣ�������ֱ��ȼ�ϵ�صĸ�����ӦΪ______________________��һ�������ѷ��Ӿ����绯ѧ���������Բ���________���ӵĵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
���仯��������Ȼ���й㷺���ڣ��������
ԭ���ش��������⣺
��1����ͼ��ʾһ���¶��£������Ϊ10L���ܱ������г���1molO2��һ������SO2��SO2��SO3(g)��Ũ����ʱ��仯�������
�ٸ��¶��£��ӷ�Ӧ��ʼ��ƽ��ʱ������ƽ����Ӧ������ ��
�ڸ��¶��£���Ӧ2SO2(g)+O2(g)  2SO3(g)��ƽ�ⳣ��Ϊ ��
2SO3(g)��ƽ�ⳣ��Ϊ ��
��2���Ի�ͭ����Ҫ�ɷ�CuFeS2��Ϊԭ�ϣ������ա�������ʹ��Ԫ�ؼ������й����ʽ���¯������ͭԪ�ػ�ԭΪͭ����������Ҫ��ӦΪ��
2Cu2S(s)+3O2(g) = 2Cu2O(s)+2SO2(g) ��H ="-768.2" kJ��mol��1
2Cu2O(s)+ Cu2S (s) = 6Cu(s)+SO2(g) ��H ="+116.0" kJ��mol��1
�١����ա�ʱ��ͨ����������ʹ��ͭ���������ɱ�ɰ����Ҫ�ɷ���Cu2S��FeS�������ʵ�����Ϊ1:2����SO2���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
���������У�Cu2S������ʵ�����O2��Ӧ����Cu���Ȼ�ѧ����ʽΪ�� ��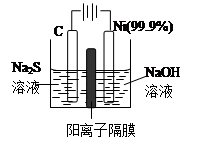
��3���õ��ķ�����������Һ����Ϊ��������о�������Ҫ��ʵ�����壬������ת��Ϊ�������ǵ�ⷨ�������������һ����Ҫ���ݡ�
��ͼ���ǵ������������ʵ��װ�ã�
����֪�����ķ�ӦΪ��(x+1)S2��=SxS2��+2xe�����������ĵ缫��Ӧʽ�ǣ� ��
����Ӧת��xmol����ʱ���������������Ϊ ����״���£���
�ڽ�Na2S��9H2O����ˮ������������Һʱ��ͨ�����ڵ����������ܽ⡣��ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С�������50 mL 1.0 mol/L�����50 mL 1.1 mol/L ����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ����(��ֽ��)��ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ����(��ֽ��)�����ձ�������ĭ���ϰ�(��Ӳֽ��)���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��Իش��������⣺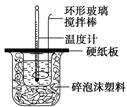
��1����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���к͡����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к��� (�ƫ����ƫС�����䡱)��
��2�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����IJ��裬���˲������裬���õ��к��Ȼ� (�ƫ����ƫС�����䡱)��
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ� (�ƫ����ƫС�����䡱)����ԭ���� ��
��4����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50 mL������¼��ԭʼ����(���±�)��
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�(t2)/�� | �²�(t2��t1)/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com