
 Ca��ClO3��2+5CaCl2+6H2O��
Ca��ClO3��2+5CaCl2+6H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�γ���������ѧ������ѧ��ѧ����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
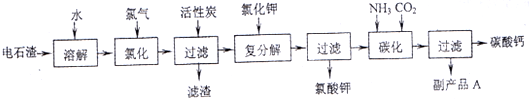
(12 ��)�Ե�ʯ��[��Ҫ�ɷ���Ca(OH)2����SiO2��Al2O3�Լ�������������]Ϊԭ���������������̼��Ƶ��������£�
�ش��������⣺
��1����ʯ������ˮ�γɵ�ʯ����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��
_____________________________________��__________________________________��
��2���Ȼ����̵��¶ȿ�����75��80�棬�ù�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ��
________________________________________________________________________��
��3���������м������̿��������_____________________________________________��
��4����������̼����Ӧ�����ӷ���ʽΪ_________________________________________��
��5������ƷA�Ļ�ѧʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
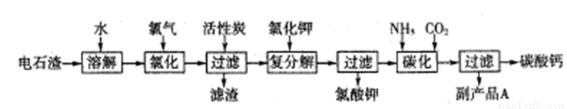
�Ե�ʯ��(��Ҫ�ɷ���Ca(OH)2����SiO2�Լ�������������)Ϊԭ�������������������̼��Ƶ�����������
�ش��������⣺
��1����ʯ������ˮ�γɵ�ʯ����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��????????????
��2���Ȼ����̵��¶ȿ�����75��80�����ù�����Ҫ��Ӧ�����ӷ���ʽΪ��?????????
��3���������м������̿��������??????????????????????????
��4��̼����������������Һ��ͨ����������ͨ��CO2��
��ʵ����ͨ�����ü����Ȼ�狀��������ƻ����ķ�����ȡ������ijѧϰС��ѡȡ��ͼ��������װ����ȡ���ռ������İ�����
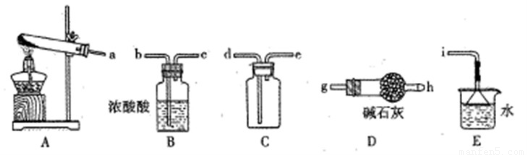
����������������Ӹ������ӿڣ�����Ϊ��ȷ��˳��Ϊa�� ? ? ��????? �� ???? ��????? ��i��������i����©����������?? ????????????? ��
��ʵ�����л����ù����������ƺ�Ũ��ˮ��ȡ�����������������ʺ���ɸ�ʵ��ļ���װ���� ??? ?? (����)

��5������ƷA�Ļ�ѧʽΪ ????????? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�γ��и�����ѧ��ѧ����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
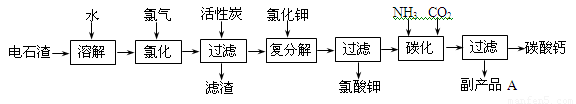
(12 ��)�Ե�ʯ��[��Ҫ�ɷ���Ca(OH)2����SiO2��Al2O3�Լ�������������]Ϊԭ���������������̼��Ƶ��������£�
�ش��������⣺
��1����ʯ������ˮ�γɵ�ʯ����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��
_____________________________________��__________________________________��
��2���Ȼ����̵��¶ȿ�����75��80�棬�ù�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ��
________________________________________________________________________��
��3���������м������̿��������_____________________________________________��
��4����������̼����Ӧ�����ӷ���ʽΪ_________________________________________��
��5������ƷA�Ļ�ѧʽΪ________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com