
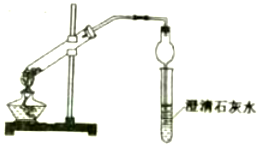
 ������ƿ��ɫ����ֱ���̼���ƹ�����̼�����ƹ��壬ijͬѧ���ö��ַ������м���
������ƿ��ɫ����ֱ���̼���ƹ�����̼�����ƹ��壬ijͬѧ���ö��ַ������м������� ��1������1��̼�����Ʋ��ȶ��������ֽ⣬���ɶ�����̼��ʹ����ʯ��ˮ����ǣ�̼���Ƽ��Ȳ��ֽ⣻��װ�ô��ڵIJ���֮���ǣ����ȵ��Թ�δ������б���ױ��ѣ�
����2����ͬ�����£���ˮ��̼���Ƶ��ܽ��С��̼�����Ƶ��ܽ�ȣ�
����3�����Ƶ�Ũ�ȵĶ��ߵ���Һ���ⶨpH��pH���ΪNa2CO3��ʵ����������Һ�IJ�������ֱ��ǣ����㡢�������ܽ⣬���ݸ���������Ҫʹ�õ����������з������
��2���μ�0.1 mol•L-1 ��������������̼ȫ��ת��Ϊ������̼����12.6g��Ʒ��̼��������̼���Ƶ����ʵ����ֱ�Ϊx��y���������ɵĶ�����̼��̼�غ㡢�����غ���
��� �⣺��1������1��̼�����������ֽ⣬��̼���Ʋ��ܣ�����ȣ�������ų�����ӦΪ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2������ʹ����ʯ��ˮ����ǣ���ӦΪ��CO2+Ca��OH��2=CaCO3��+H2O���ɼ��𣬼���̼�����Ʒֽ�����ˮ�����ȵ��Թ�δ������б���ױ��ѣ�
�ʴ�Ϊ��NaHCO3�����ȵ��Թ�δ������б���ױ��ѣ�
����2����ͬ�����£��������̼�������ܽ��С��̼���Σ�����̼�����Ƶ��ܽ��С��̼���ƣ�����ʣ������С����Na2CO3��
�ʴ�Ϊ��Na2CO3��
����3��̼������ǿ�������Σ���Һ��̼�������ˮ���Լ��ԣ�CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-����ҺpH��7��̼������ֻ����HCO3-+H2O?H2CO3+OH-����������pH�����Na2CO3��������Һ�����ȼ���������Һ�������ʺ�ˮ���������ٳ�����������ʺ���ȡˮ���������ܽ⡢װƿ��ҩ������ȡ�ù���ҩƷ��������ƽ���ڳ�ȡ�������ʣ���Ͳ�뽺ͷ�ι�����ȷ��ȡˮ���ձ���������ܽ�����������������ܽ�ʱ�Ľ��裬���װ���Լ�ƿ������Ҫ�Ķ���������������ƽ����Ͳ��
�ʴ�Ϊ��Na2CO3����Ͳ��
��2����12.6g��Ʒ��̼��������̼���Ƶ����ʵ����ֱ�Ϊx��y������������ɵã�84g/mol•x+106g/mol•y=12.6g������CO2���3.36L����״����Ϊ0.15mol������̼�غ�x+y=0.15���������̣��⣬0.15molCO2����ȫ��������̼���ƣ�0.15mol��84g/mol=12.6g���ʰ�ɫ������ƷΪһ�ֳɷ�Ϊ̼���ƣ�
�ʴ�Ϊ��̼���ơ�12.6g��
���� ������̼���ƺ�̼������Ϊ���忼�����ʵļ���Ϊ��Ƶ���㣬�������ʵ����ʲ���Ϊ���Ĺؼ���ע�����ò�ͬ����������ʣ���Ŀ�Ѷ��еȣ�


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������NaOH����ʱ���ձ����ܽ������ת�Ƶ�����ƿ�� | |
| B�� | ������ƿ�м�ˮ����ʱ���� ������ҺŨ��ƫ�� | |
| C�� | ����NaOH��Һ�����õ���Ͳ | |
| D�� | ���ݺ�ҡ�ȣ�����Һ�潵�ͣ��ֲ�������ˮ�����´ﵽ�̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶��W��Z��Y | |
| B�� | W������������Ӧˮ���������ǿ��Z������������Ӧˮ��������� | |
| C�� | X��Z�γɵļ������X��W�γɵļ������ȶ� | |
| D�� | X��Y�γɵĻ�����һ��ֻ�����Լ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��AgNO3��Һ�м���Cu�ۣ�Cu+Ag+�TCu2++2Ag | |
| B�� | ��CaCl2��Һ��ͨ��CO2��Ca2++CO2�TCaCO3��+2H+ | |
| C�� | ����FeCl��Һ�����ˮ����Fe��OH��3���壺Fe3++3H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3H+ | |
| D�� | CuSO4��Һ��Ba��OH��2��Һ��ϣ�Ba2++SO42-�TBaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����ʵ | ���۽��� |
| A | SO2����ˮ�γɵ���Һ�ܵ��� | SO2�ǵ���� |
| B | ����Ϊ����������� | ��������P-P���ļ�����109.5�� |
| C | 1���ˮ�����ܽ�700������� | ���Ǽ��Է����������������Ӱ�� |
| D | HF�ķе����HCI | H-F�ļ�����H-CI�ļ����� |
| A�� | SO2����ˮ�γɵ���Һ�ܵ���SO2�ǵ���� | |
| B�� | ����Ϊ����������Ӱ�������P-P���ļ�����109.5�� | |
| C�� | 1���ˮ�����ܽ�700����������Ǽ��Է����������������Ӱ�� | |
| D�� | HF�ķе����HCIH-F�ļ�����H-CI�ļ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
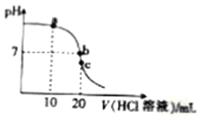
| A�� | a���Ӧ����Һ�У�c��Cl-����c��NH4+ ����c��OH- ����c��H+ �� | |
| B�� | b���Ӧ����Һ�У�c��NH4+����c��Cl-����c��OH- ��=c��H+ �� | |
| C�� | c���Ӧ����Һ�У�c��H+��=c��NH3•H2O��+c��OH- �� | |
| D�� | �ζ������п��ܳ��֣�c��NH4+ ����c��OH- ����c��H+ ����c��Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����5 mL ����ʱ��c��NH4+����c��Cl-����c��OH-����c��H+�� | |
| B�� | ����10mL ����ʱ��c��NH4+��+c��H+��=c��OH-��+c��Cl-�� | |
| C�� | ����ҺpH=7 ʱ��ˮ�ĵ���̶���� | |
| D�� | ����20 mL ����ʱ��c��Cl-��=2c��NH4+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 7.8g | B�� | 15.6g | C�� | 3.9g | D�� | 78g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com