
Ϊ�ⶨij��������Na2O��Na2O2��Ʒ�Ĵ��ȣ�3��С��ֱ�������·���������ȷ������Ʒmg��Ȼ�������·�������ʵ�飺
[����һ]������Ʒ��ˮ��ַ�Ӧ��ʹ������O2ͨ�����ȵ�ͭ�ۣ���÷�Ӧ����������ͭ������Ϊng��ͨ���������������Na2O2�ĺ������˷����ⶨ�Ľ�����ϴ���Ҫԭ���ǣ�________��
[������]������Ʒ�������̼��Ӧ��ͨ���ⶨ��Ӧ����������������������Ʒ��Na2O2�ĺ�����
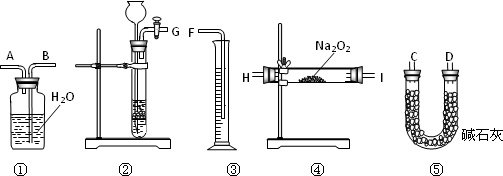
(1)���˷�����������ȡCO2��ʵ��ʹ�õ����������Ӵ�����________��(��д�������)
(2)װ�âݵ������ǣ�________��
[������]���ⶨ��Ʒ��ˮ��ַ�Ӧ����Һ�����V mL���ٴ���ȡV��1 mL��Һ��װ����ƿ���ñ�Ũ�ȵ�������еζ���ȷ����Һ��Ũ�ȣ��ټ������Ʒ��Na2O2�ĺ�����
(1)�˷��������ζ�ʱ��ѡ�õĵζ���Ϊ________(���������)��
(2)���ü�����ָʾ�����ﵽ�ζ��յ�ʱ������Ϊ________������Ϊ���������������вⶨ����Ƚ�ȷ����________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
I��ij�к͵ζ�ʵ��������£�
��1��ȡһ֧������ˮϴ������ʽ�ζ��ܣ��������������Һ����¼��ʼ����
��2���ü�ʽ�ζ��ܷų�һ��������Һ������δ�ô���Һ��ϴ����ƿ�У������̪2��
��3���ζ�ʱ���ߵμӱ���ͬʱע�ӵζ�����Һ��ı仯
��4�����ε���Һ�ɺ�ɫ��Ϊ��ɫ����ɫ�ȶ���ֹͣ�ζ�����¼Һ�����
��ѡ������ʵ������еĴ���֮�� (�����)��
II��ij�ռ���Һ�к�����������(�������ᷴӦ)������������Һ�ⶨ��Ũ�ȡ�
��1���ζ�����ͼ��ʾij�εζ�ʱ50 mL��ʽ�ζ�����ǰ��Һ���λ�ã��뽫������������±��ո��С�
| �ζ���� | ����Һ��� ��mL) | ���������������mL)���ζ�ǰ�� | ���������������mL)���ζ��� | ���������������mL) |
| 1 | 25.00 | 0.50 | 25��12 | 24��62 |
| 2 | 25.00 |
|
|
|
| 3 | 25.00 | 6��00 | 30��58 | 24��58 |
��2������Ũ��Ϊ0.1000mol/L�������������ݣ�������Ʒ���ռ�����ʵ���Ũ��c = ��
��3�����м�����������ռ�Ũ����ʵ��Ũ����ȣ�(���Ӱ�족����ƫ�ߡ�����ƫ�͡�)
a�����ζ�ǰ������ˮ��ϴ��ƿ����ⶨ��� ��
b����ʽ�ζ��ܶ���ʱ�����ζ�ǰ���ӣ��ζ����ӣ���ⶨ��� ��
c����ʽ�ζ����еζ�ǰ�����ݣ��ζ���������ʧ����ⶨ��� ��
III����25mL 0.1mol/L NaOH ��Һ�м���25mL 0.2mol/L CH3COOH��Һ����ַ�Ӧ��
��pH��7������Һ����������Na+��OH-��CH3COO-��H+��CH3COOH��Ũ���ɴ�С��˳��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�������ʡ�����и���12���¿������ۣ���ѧ���� ���ͣ�ʵ����
I��ij�к͵ζ�ʵ��������£�
��1��ȡһ֧������ˮϴ������ʽ�ζ��ܣ��������������Һ����¼��ʼ����
��2���ü�ʽ�ζ��ܷų�һ��������Һ������δ�ô���Һ��ϴ����ƿ�У������̪2��
��3���ζ�ʱ���ߵμӱ���ͬʱע�ӵζ�����Һ��ı仯
��4�����ε���Һ�ɺ�ɫ��Ϊ��ɫ����ɫ�ȶ���ֹͣ�ζ�����¼Һ�����
��ѡ������ʵ������еĴ���֮�� (�����)��
II��ij�ռ���Һ�к�����������(�������ᷴӦ)������������Һ�ⶨ��Ũ�ȡ�
��1���ζ�����ͼ��ʾij�εζ�ʱ50 mL��ʽ�ζ�����ǰ��Һ���λ�ã��뽫������������±��ո��С�
| �ζ���� | ����Һ��� ��mL) | ���������������mL)���ζ�ǰ�� | ���������������mL)���ζ��� | ���������������mL) |
| 1 | 25.00 | 0.50 | 25��12 | 24��62 |
| 2 | 25.00 | | | |
| 3 | 25.00 | 6��00 | 30��58 | 24��58 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ�߶������ҵ��һ����ѧ�Ծ��������棩 ���ͣ�ʵ����
��ѧʵ�����о��������ʵĻ�����
(1)�����й�ʵ�������������ݺ�������________(�����)��
a��������������CuSO4��5H2O����ⶨ�ᾧˮ��������
b���ø����pH��ֽ�ⶨŨ�����pH
c���ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ
(2)ij��ˮ��Ʒ�к���һ������Na����CO32-��SO32-��ij�о�С�����ⶨ����SO32-��Ũ�ȡ�
ʵ�鷽����
��.���ձ�ʢȡ��ˮ����������������̿����ȥ��ˮ�е����ʣ����ˣ�ȡ��Һ��
��.��ȷ��ȡ20.00 mL���˺��ˮ������ѡ��ʹ����ɫ��0.1 mol/L KMnO4(H2SO4�ữ)��Һ���еζ���
��.��¼���ݣ����㡣
�����еζ���ʽ�У����������(�гֲ��ѷ���ȥ)______(����ĸ���)��

�ڵζ������У��йط�Ӧ�����ӷ���ʽ��__________________________________��
(3)ijͬѧ�Ʊ�Fe(OH)3���壺�ýྻ���ձ�ȡ��������ˮ���������ڣ����ձ��еμ� 1 mol/L��FeCl3��Һ���������ò��������裬�����Һ����ǡ���ͬѧ�Ʊ�����ʧ�ܵ�ԭ������������������������Ϊ�ɹ��Ƶ�Fe(OH)3���������������_________��
(4)����ͼװ�ý���CO2���ʵ��й�ʵ�顣

�Լ�ƿB��ʢ�б���NaHCO3��Һ����Ŀ����:
_______________________ __________��
�ڷ�Ӧ�����У�E�г���ʯ��ˮ����ǣ�E�еĻ����ϵ�г����ڵ���ƽ�⡢ˮ��ƽ���⣬�������ܽ�ƽ�⣬�÷���ʽ��ʾ���ܽ�ƽ���ϵ��
____________________ ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������ʡ����12���¿������ۣ���ѧ���� ���ͣ�ʵ����
I��ij�к͵ζ�ʵ��������£�
��1��ȡһ֧������ˮϴ������ʽ�ζ��ܣ��������������Һ����¼��ʼ����
��2���ü�ʽ�ζ��ܷų�һ��������Һ������δ�ô���Һ��ϴ����ƿ�У������̪2��
��3���ζ�ʱ���ߵμӱ���ͬʱע�ӵζ�����Һ��ı仯
��4�����ε���Һ�ɺ�ɫ��Ϊ��ɫ����ɫ�ȶ���ֹͣ�ζ�����¼Һ�����
��ѡ������ʵ������еĴ���֮�� (�����)��
II��ij�ռ���Һ�к�����������(�������ᷴӦ)������������Һ�ⶨ��Ũ�ȡ�
��1���ζ�����ͼ��ʾij�εζ�ʱ50 mL��ʽ�ζ�����ǰ��Һ���λ�ã��뽫������������±��ո��С�

|
���� |
����Һ��� ��mL) |
���������������mL)���ζ�ǰ�� |
���������������mL)����� |
���������������mL) |
|
1 |
25.00 |
0.50 |
25��12 |
24��62 |
|
2 |
25.00 |
|
|
|
|
3 |
25.00 |
6��00 |
30��58 |
24��58 |
��2������Ũ��Ϊ0.1000mol/L�������������ݣ�������Ʒ���ռ�����ʵ���Ũ��c = ��
��3�����м�����������ռ�Ũ����ʵ��Ũ����ȣ�(���Ӱ�족����ƫ�ߡ�����ƫ�͡�)
a�����ζ�ǰ������ˮ��ϴ��ƿ����ⶨ��� ��
b����ʽ�ζ��ܶ���ʱ�����ζ�ǰ���ӣ��ζ����ӣ���ⶨ��� ��
c����ʽ�ζ����еζ�ǰ�����ݣ��ζ���������ʧ����ⶨ��� ��
III����25mL 0.1mol/L NaOH ��Һ�м���25mL 0.2mol/L CH3COOH��Һ����ַ�Ӧ��
��pH��7������Һ����������Na+��OH-��CH3COO-��H+��CH3COOH��Ũ���ɴ�С��˳��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com