
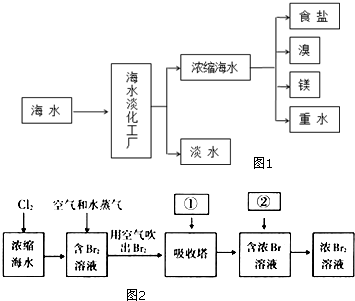
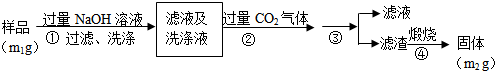
 ��嫵ĺ�����һ�������Դ���⣬�̲��ŷ��ĵĿ�����DZ���Ļ�ѧ��Դ����ͼ1�Ǻ�ˮ�ӹ���ʾ��ͼ��������ͼ�ش����⣮
��嫵ĺ�����һ�������Դ���⣬�̲��ŷ��ĵĿ�����DZ���Ļ�ѧ��Դ����ͼ1�Ǻ�ˮ�ӹ���ʾ��ͼ��������ͼ�ش����⣮���� ��1��Ŀǰ������ˮ�ķ����ж��֣����÷��������������Լ����ӽ������ȣ�
��2����Ũ����ˮ����ȡ�壬�Ƚ��������ӵ������õ��嵥�ʣ����ö������������嵥�ʸ����õ������ӣ�����ٴ�������������
��3��MgO���۵�Ϊ2852�桢�۵�ߣ����ʱ���ܸߣ�
��4��ʳ��ˮ�к�����������Mg2+��Ca2+��һ�����������ɳ��������ӽ���Ĥ�����ݵ��ʳ��ˮ�IJ���֮���ܷ�Ӧ��
��5���������η�����������ƽ��ʱH2��SiHCl3���ʵ���Ũ�ȣ��������ʼ���ʵ������ٸ���2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2NaOH��������������Ĵ�NaCl��������
��� �⣺��1��Ŀǰ������ˮ�ķ����ж��֣���ˮ��������ͨ�����õ��Ʊ���ˮ�ķ����У���������������
�ʴ�Ϊ����������������
��2����Ũ����ˮ����ȡ�壬�Ƚ��������ӵ������õ� �嵥�ʣ����ö������������嵥�ʵĸ����õ������ӣ�����ٴ��������������������з����Ļ�ѧ��Ӧ����ʽΪ��SO2+Cl2+2H2O=H2SO4+2HCl��
�ʴ�Ϊ��SO2��Cl2��SO2+Cl2+2H2O=H2SO4+2HCl��
��3��MgO���۵�Ϊ2852�桢�۵�ߣ����ʱ���ܸߣ����Թ�ҵ�ϣ��������MgCl2ұ������þ��
�ʴ�Ϊ��MgO�۵�ܸߣ������Ĵ������ܣ�
��4�����Լ����������ˮ�л���������Mg2+��Ca2+�����������»����ɳ����������ӽ���Ĥ������Ҫ�������ӽ�������ȥMg2+��Ca2+�����ʳ��ˮ�IJ������������������������ƣ����������������ܷ�Ӧ������������Ҳ�ܷ�Ӧ�����Ե��ʳ��ˮ�����ӽ���Ĥ�����н��У�
�ʴ�Ϊ�����Լ����������ˮ�л���������Mg2+��Ca2+�����������»����ɳ����������ӽ���Ĥ����ֹH2��C12��Ϸ�����ը��ͬʱ��ֹC1-���������ң��������Ի�ô�����NaOH��
��5����Ӧ����ʽ��֪��3SiCl4��g��+2H2��g��+Si��s��?4SiHCl3��g��
��ʼ����mol�� n 0
�仯����mol�� 2x x 4x
ƽ������mol�� n-2x 4x
��4x=0.020mol/L��20L=0.4mol��x=0.1mol��
n-2x=0.140mol/L��20L=2.8mol��n=3.0mol��
��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2NaOH��
2mol 1mol
$\frac{2��58.5g}{1mol}$=$\frac{m��NaCl��}{3mol}$��
��ã�m��NaCl��=350g=0.35kg��
�ʴ�Ϊ��0.35��
���� ���⿼���˺�ˮ��Դ���ۺ����ã���Ŀ�Ѷ��еȣ�Ϊ�߿��������ͣ���һ���ۺ������⣬�漰֪ʶ��Ƚ϶࣬��ֿ�����ѧ�����Ӧ�û���֪ʶ��������Ҫ��ѧ����������֪ʶ�ṹ�ͷ��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ԫ��A���γɵ��⻯�ﳣ����һ��Ϊ��̬ | |
| B�� | �ǽ����ԣ�A��E | |
| C�� | Ԫ��C��D��E����Ȼ���о�����������̬���� | |
| D�� | Ԫ��B���������Ӧ��ˮ����һ��Ϊǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij������Һ��pH=a��������Һϡ��10������Һ��pHֵΪb����b=a+1 | |
| B�� | CH3OOH��CH3COONa�����Һ�����ܴ��ڣ�c ��CH3COOH����c ��CH3COO-����c ��H+����c ��Na+����c ��0H-�� | |
| C�� | �����£���pH=3��H2SO4��pH=11��һԪ��BOH-��Һ�������ϣ�������Һ����Ϊ���Ի����� | |
| D�� | �����£�Ũ�Ⱦ�ΪO��1mol•L-1�Ģ�CH3COOH��Һ��NaOH��Һ��CH3OONa ��Һ�У�ˮ�ĵ���̶Ȣۣ��٣��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
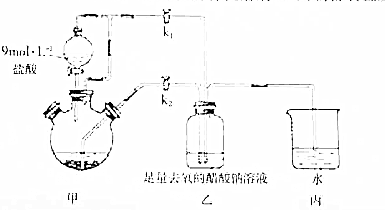 �ټ��װ�������Ժ���������ƿ�����μ������п��������CrCl3��Һ��
�ټ��װ�������Ժ���������ƿ�����μ������п��������CrCl3��Һ�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������E��F | |
| B�� | �����ʵ�����E��F�ֱ�������ϡ���ᷴӦ���������������ʵ�����E��F | |
| C�� | Ea+��Fb+����ͬ�ĵ��Ӳ�ṹ��a��b�� | |
| D�� | 25��ʱ��Ksp[E��OH��a]��Ksp[F��OH��b] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
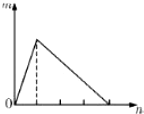 ����Һ�������壩X���루��ͨ�룩��һ����Y��Һ�У�������������m�����X�����ʵ���n�Ĺ�ϵ��ͼ��ʾ������ͼ�������һ�������ǣ�������
����Һ�������壩X���루��ͨ�룩��һ����Y��Һ�У�������������m�����X�����ʵ���n�Ĺ�ϵ��ͼ��ʾ������ͼ�������һ�������ǣ�������| A | B | C | D | |
| X | Ba��OH��2 | NaOH | NH3 | HCl |
| Y | ���� | AlCl3 | MgSO4 | NaAlO2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com