
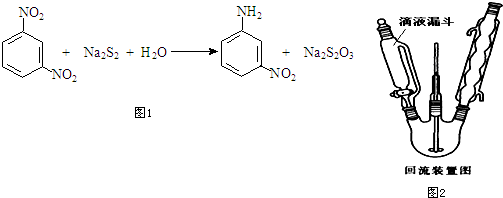
���� ��1����ѹ��Һ©�������Һ©������ƽ������ѹǿ������ʹ������ƿ��ѹǿ��©���е�ѹǿ��ȣ�ʹ��������Һ˳�����£�����һ����Һʱ���ձ����ɣ�
��2���ж����ʻ�����ж����ʵ�ʵ����Ҫ��ͨ����н��У�
��3���л�����ȥ������Ϊ��ԭ��Ӧ�����������Ϣ�������������Ƶó���̬��ԭ�ӣ���ԭ��Զǿ�ڼ�������������ԭ��ǿ��������ѡ���ԣ�δ���ܽ�������ԭΪ�������ò�����Ҫ�IJ�Ʒ��
��4������������Ϊ�л������ˮ���ڢܲ��������ܽ�ֲ�Ʒ�����������������������ÿ����γ�������ˮ�У�
��5���˱�ϴ�����Σ�ϴȥ��������ʣ�������Ϣ֪��������������ˮ�����¶������ܽ�����������Ҵ������ѡ��״�������ѡ����ˮϴ�ӣ��ڢ�����Ҫ�����ؽᾧ���轫�ֲ�Ʒ�ܽ⣬����ˮ���������л���������ؽᾧ��
��6���ڢ۲��У�����ʹ�����������ˮ�γ�����Һ���ٵμӶ�������Һ������Ӵ�������ӿ췴Ӧ���ʣ����ԭ�������ʣ�
��7��������ҺΪ���ԣ���PH��ֽ���飬ȡ���һ��ϴ��Һ�����������ԣ�˵���˱�ϴ�ӵ����ԣ�
��8�����ݲ���=$\frac{ʵ�ʲ�Ʒ����}{���۲�Ʒ����}$��100%���㣮
��� �⣺��1������ͨ��Һ©����ȣ���ѹ��Һ©���ϲ���������ƿ��ѹ��ͨ�����Ա�֤��ѹ��Һ©���е�Һ��˳�����£�������Һ��Ҫ���ձ��н��У�
�ʴ�Ϊ�����ֺ�ѹ������Һ��˳�����£��ձ���
��2���ж����ʻ�����ж����ʵ�ʵ����Ҫ��ͨ����н��У���ֹ��ȫ�¹ʲ������ʴ�Ϊ��ͨ�����
��3��������������ѡ�ü�����������������������ѡ���Ի�ԭ���������������Ƶó���̬��ԭ�ӣ���ԭ��Զǿ�ڼ�������������ԭ��ǿ��������ѡ���ԣ�δ���ܽ�������ԭΪ�������ò�����Ҫ�IJ�Ʒ�����Բ�ѡ��
�ʴ�Ϊ�������������û�ԭ��ǿ��������ѡ���ԣ�
��4���ڢܲ��������ܽ�ֲ�Ʒ������Ϊ���������������������ÿ����γ�������ˮ�У�����ˮ��ԭ���Ǽ�������������ˮ��
�ʴ�Ϊ����������������ˮ���������������ÿ����γ�������ˮ�У�
��5����������������ˮ�����¶������ܽ�����������Ҵ������ѡ��״�������ѡ����ˮϴ�ӵڢ۲����˱����Σ����νᾧҲ�����ؽᾧ�����ᴿ���壬���������������Ҵ������ѡ��״����������ؽᾧ������ˮ��ͨ���¶ȵ��ڣ����ý��½ᾧ�ᴿ���壬����ѡ����ˮ��
�ʴ�Ϊ��A��B��
��6���ڢ۲��У�����ʹ�����������ˮ�γ�����Һ�ٵμӶ�������Һ��������Ӧ���ĽӴ�������ӿ췴Ӧ���ʣ����ԭ�������ʣ�
�ʴ�Ϊ������Ӵ�������ӿ췴Ӧ���ʣ����ԭ�������ʣ�
��7���˱�ϴ���Ƿ����ԣ���ͨ���������ϴ��Һ������֤���ò�����պȡ���һ��ϴ�Ӻ�Һ�壬����pH��ֽ���룬�ⶨpH����pH=7��˵����ϴ�ӵ����ԣ�
�ʴ�Ϊ���ò�����պȡ���һ��ϴ�Ӻ�Һ�壬����pH��ֽ���룬�ⶨpH����pH=7��˵����ϴ�ӵ����ԣ�
��8��4.74g�����������Mr=158��������ת��Ϊ���������������ʵ���Ϊ$\frac{4.74g}{158g/mol}$�������Ƶü���������������Ϊ��$\frac{4.74g}{158g/mol}$��128g/mol=3.84g�������ؽᾧ�ᴿ���ں�����¸�����أ���2.56g������=$\frac{ʵ�ʲ�Ʒ����}{���۲�Ʒ����}$��100%=$\frac{2.56g}{3.84g}$��100%��66.7%��
�ʴ�Ϊ��66.7%��
���� ���⿼������������Ʊ�ʵ�鷽�����漰���ʵķ����ᴿ����װ��������ķ�������ѧ����ȣ���ȷԭ���ǽ���ؼ�����Ҫѧ���߱���ʵ�Ļ������л���ѧʵ������ѧ�н��٣�ѧ����İ�������¸о������������裬��Ŀ�Ѷ��еȣ�


 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Һ̬�廯�� | B�� | Һ�� | C�� | �� | D�� | ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij��������Ϊ2��3��4-����-3��5-���һ����� | |
| B�� | �ṹƬ��Ϊ �ĸ߾�����䵥��ͨ�����۷�Ӧ���� �ĸ߾�����䵥��ͨ�����۷�Ӧ���� | |
| C�� | ���е��ǡ���֬�������ʶ���ˮ�⣬��ˮ����ﲻͬ | |
| D�� | �����Ӳ֬�ụΪͬϵ�C2H6��C9H20Ҳһ����Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ��ɫ��״̬ | �е㣨�棩 | �ܶȣ�g•cm-3�� | |
| ������* | ��ɫ��Ƭ״���� | 249 | 1.2659 |
| ���������� | ��ɫ����Һ�� | 212.6 | 1.05 |
| �Ҵ� | ��ɫ����Һ�� | 78.3 | 0.7893 |
| ������ | ��ɫ����Һ�� | 80.8 | 0.7318 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����£���20ml 0.1mol/L ��ʯ�ᣨ��H2T��ʾ����Һ���μӵ����ʵ���Ũ�ȵ�NaOH��Һ���й��������ʵ���������Һ��pH����ͼ��ϵ������˵����ȷ���ǣ�������
�����£���20ml 0.1mol/L ��ʯ�ᣨ��H2T��ʾ����Һ���μӵ����ʵ���Ũ�ȵ�NaOH��Һ���й��������ʵ���������Һ��pH����ͼ��ϵ������˵����ȷ���ǣ�������| A�� | HT-����Һ��ˮ��̶ȴ��ڵ���̶� | |
| B�� | ��V��NaOH��=20mLʱ����Һ��ˮ�ĵ���̶ȱȴ�ˮ�� | |
| C�� | ��V��NaOH��=30mLʱ����Һ�д���c��Na+����c��HT-����c��T2-����c��OH-����c��H+�� | |
| D�� | ��V��NaOH��=40mlʱ����Һ�д���c��OH-��=c��HT-��+2 c��H2T��+c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ԫ�ص�ԭ�Ӱ뾶��С�����˳��Ϊ��r��X����r��Z����r��W����r��Y���� | |
| B�� | X��Y��Z�����γ����ӻ���������γɹ��ۻ����� | |
| C�� | X����������Ԫ��֮���γɵĺ����������Ϊ10����ֻ��2�� | |
| D�� | H2Z���۵��H2W�ߣ�����Ϊ���ǵľ������Ͳ�ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��¼Ƭ��֮�¡��е���������С���������ۺ�����ǿ����ö����������� | |||||||
| B�� | ��װú̿��������������װ�ã�������Ч���⡰�����ֵĸ��� | |||||||
| C�� | �ҹ���ú�����ٵĹ��ң�ú�����׳���˹������һ�������õ���Դ��ú�������ֱ����ú����࣬���ڶԿ����� | |||||||
| D�� | �±�Ϊȫ����Ҫ��������2014��PM2.5��ֵ��PM2.5���ֲ���Ϊ̼��άձ�桢�߷���֯��IJ��ȣ���һ�ּ�϶�Ȱ�Ĥ��С�ķ���ɸ��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���� | ||
| C�� | �ð�Ĥ�������� | D�� | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com