
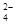 ��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�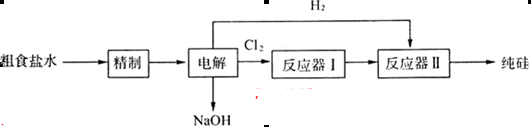
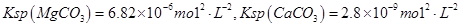 ������ô���ˮ��
������ô���ˮ�� ��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��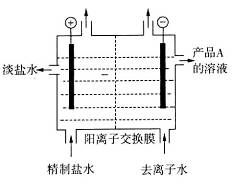
 Si+2CO����2�֣�
Si+2CO����2�֣� Si+4HCl��2�֣�
Si+4HCl��2�֣� NaClO+H2����2�֣�
NaClO+H2����2�֣� Si+2CO��
Si+2CO�� Si+4HCl��
Si+4HCl�� NaClO+H2��
NaClO+H2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

A�������ճ�ɰ���������е���ת�Ƶķ������Ŀ�ɱ�ʾΪ��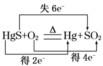 |
| B����ɰ�������Ƽ��ȷ�Ӧʱ��CaSO4Ϊ�������� |
| C��ϴ�Ӵֹ�����5%���������5%������ |
| D����ѹ�����Ŀ���ǽ����ķе㣬��߷���Ч�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
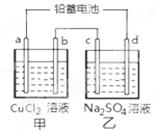
| A��d������ |
| B�������ü׳ؾ���ͭ��b��ӦΪ��ͭ |
| C���ŵ�ʱǦ���ظ����ĵ缫��ӦʽΪ�� PbO2(s) �� 4 H��(aq)��SO4 2��(aq)��4e�� = PbSO4 (s) ��2H2O (l�� |
| D�����ĸ��缫���Ͼ�Ϊʯī��������6��4 g Cuʱ�������й���������3��36 L(��״����) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1 mol��L��1 | B��2 mol��L��1 | C��3 mol��L��1 | D��4 mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
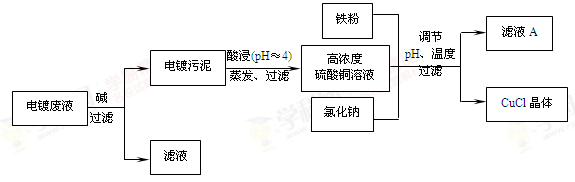
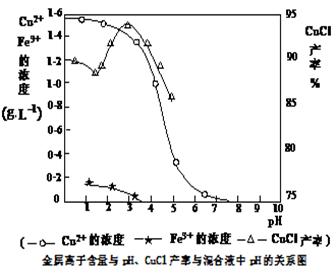
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
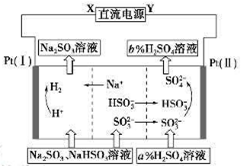
| A��XΪֱ����Դ��������YΪֱ����Դ�ĸ��� |
| B��������pH��С |
| C��ͼ�е�b��a |
| D�������ĵ缫��ӦΪHSO3����2e����H2O��SO42����3H���� SO32����2e����2H2O��SO42����4H�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1mol/L | B��2mol/L | C��2.5mol/L | D��3mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
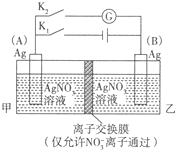
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
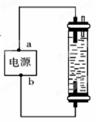
| A��a������b���� | B��a������b���� |
| C��a������b���� | D��a������b���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com