
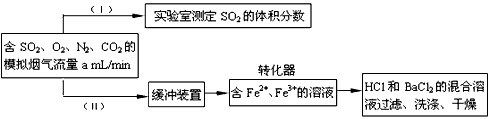
 SO2(g)+1/2O2(g)��ƽ�ⳣ����д��������̣�__________________________
SO2(g)+1/2O2(g)��ƽ�ⳣ����д��������̣�__________________________  ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ���� | ���Ӱ뾶��pm�� | ��ʼ����pH | ��ȫ����pH |
| Fe2+ | 74 | 7.6 | 9.7 |
| Fe3+ | 64 | 2.7 | 3.7 |
| Al3+ | 50 | 3.8 | 4.7 |
| Mn2+ | 80 | 8.3 | 9.8 |
| Pb2+ | 121 | 8.0 | 8.8 |
| Ca2+ | 99 | - | - |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ʯ���� | ��ͭ�� | ��ͭ�� | ��ͭ�� | ��ȸʯ |
| ��Ҫ�ɷ� | CuFeS2 | Cu5FeS4 | Cu2S | CuCO3?Cu��OH��2 |
| ||
| ѡ�� | ������ | ������ | �ж� |
| A | ͭ�̵����ɷ��Ǽ���ͭ | ����ϡ�����ͭ�������ͭ�� | ��ԣ���ԣ��� |
| B | ͭ�����γ����ܵ�����Ĥ | ͭ��������ʢ��Ũ���� | ��ԣ���ԣ��� |
| C | ����ͭ���� | ����ͭ���ϵ������ڳ�ʪ�����в������� | ��ԣ���ԣ��� |
| D | ��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 | ����ͭ��Һ��������Ӿ�ص������� | �������ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
![]() ͭ����Ȼ������ڶ��ֿ�ʯ�У��磺
ͭ����Ȼ������ڶ��ֿ�ʯ�У��磺
| ��ʯ���� | ��ͭ�� | ��ͭ�� | ��ͭ�� | ��ȸʯ |
| ��Ҫ�ɷ� | CuFeS2 | Cu5FeS4 | Cu2S | CuCO3��Cu(OH)2 |
��ش��������⣺
��1���ϱ�����ͭ�������У�ͭ�������ٷֺ�����ߵ���������������
��2����ҵ���Ի�ͭ��Ϊԭ�ϡ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O+Cu2S![]() 6Cu+SO2������Ӧ����������������������
6Cu+SO2������Ӧ����������������������
��3��SO2β��ֱ���ŷŵ���������ɻ�����Ⱦ�ĺ����������������������β���ɵõ��м�ֵ�Ļ�ѧƷ��д������1�����1���ε�����������������
��4����ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ơ���д����ͭ���õ���ͭ�ĵ缫��Ӧʽ������������ ���������� ��
��5���±��У��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ����������������ĸ����
| ѡ�� | ������ | ������ | �ж� |
| A | ͭ�̵����ɷ��Ǽ���ͭ | ����ϡ�����ͭ�������ͭ�� | ��ԣ���ԣ��� |
| B | ͭ�����γ����ܵ�����Ĥ | ͭ��������ʢ��Ũ���� | ��ԣ���ԣ��� |
| C | ����ͭ���� | ����ͭ���ϵ������ڳ�ʪ�����в������� | ��ԣ���ԣ��� |
| D | ��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 | ����ͭ��Һ��������Ӿ�ص������� | �������ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ̩���и����ڶ��ָ�ϰ����������ۻ�ѧ�Ծ��������棩 ���ͣ������
��ҵ�ϳ����õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)�����պ�SO2�ķ����������Ƶ������̾���(MnSO4��H2O)������Ҫ�������£�
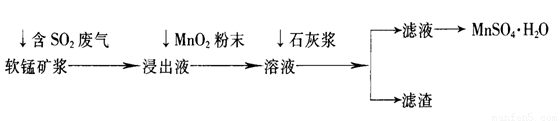
��֪������Һ��pH��2�����еĽ���������Ҫ��Mn2+��������������Fe2+��Al3+�������������ӡ��йؽ��������γ������������ʱ��Һ��pH���±���
|
���� |
��ʼ����ʱ��pH |
��ȫ����ʱ��pH |
|
Fe2+ |
7.6 |
9.7 |
|
Fe3+ |
2.7 |
3.7 |
|
Al3+ |
3.8 |
4.7 |
|
Mn2+ |
8.3 |
9.8 |
��1�����̿���ͨ�뺬SO2����������Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________________��
��2������Һ�м���MnO2��ĩ��Ŀ����____________����Ӧ�����ӷ���ʽ��___________________��
��3����Һ�м���ʯ�ҽ�������pH����������_________________��pHӦ���ڵķ�Χ��__________��
��4����������Ҫ�ɷ���________________________________________________(�û�ѧʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com