
���������Ȼ�ѧ����ʽ�У��йئ�H�ıȽ���ȷ����( )
��CH4(g)��2O2(g)===CO2(g)��2H2O(g) ��H1 CH4(g)��2O2(g)===CO2(g)��2H2O(l) ��H2
��NaOH(aq)��H2SO4(Ũ)===Na2SO4(aq)��H2O(l) ��H3
NaOH(aq)��CH3COOH(aq)===CH3COONa(aq)��H2O(l) ��H4
A����H1>��H2����H3>��H4 B����H1>��H2����H3<��H4
C����H1����H2����H3<��H4 D����H1<��H2����H3<��H4
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������ĵ����һ�и߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���л���ķ����У���ij��̼ԭ���������ĸ���ͬ��ԭ�ӻ�ԭ���ţ�����̼ԭ�ӳ�Ϊ������̼ԭ�ӡ�������һ������̼ԭ�ӵ�����һ�����й�ѧ���ԣ���ͼ�����й�ѧ���ԣ��������з�Ӧ�����ɵ��л������й�ѧ���Ե���

�������ᷢ��������Ӧ ����NaOHˮ��Һ����
����������Һ���� ���ڴ���������������H2���� ����CuO����
A���� B���٢ڢܢ� C�� �ۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ����Ϻ���һ�и�һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
X��Y��Z��W��������Ԫ�أ���X����������Y�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��W�������ӵ�������ǿ�ڵȵ������X�����ӵ������ԣ�Z�����Ӱ뾶���ڵȵ������Y�������Ӱ뾶��������Ԫ�ص�ԭ�������ɴ�С����˳����( )
A��Z��X��Y��W B��W��X��Y��Z C��X��Z��Y��W D��Z��Y��X��W
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijͬѧ����ϡ������п��ȡ������ʵ���У����ּ�����������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
��1������ͭ��Һ���Լӿ������������ʵ�ԭ����_______________________��
��2��ʵ����������Na2SO4��MgSO4��Ag2SO4��K2SO4��4����Һ����������ʵ����CuSO4��Һ���������õ���____________��
��3��Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ��_____________________(������)��
��4��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬��ͬѧ���������һϵ��ʵ�顣�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������塣��¼�����ͬ�������������ʱ�䡣
ʵ�� �����Һ | A | B | C | D | E | F |
4 mol��L��1 H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
������ɴ�ʵ����ƣ����У�V1��________��V6��________��V9��______��
�ڸ�ͬѧ���ó��Ľ���Ϊ������������CuSO4��Һʱ���������������ʻ�����ߡ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��Pt�缫��⺬�и�0.1 mol Cu2����X3������Һ�����������������ʵ�����m(g)����Һ��ͨ�����ӵ����ʵ���n(mol)�Ĺ�ϵ��ͼ��ʾ�������ӵ����������ɴ�С������ȷ����( )
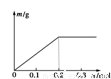
A��Cu2����X3����H��
B��H����X3����Cu2��
C��X3����H����Cu2��
D��Cu2����H����X3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��N2��H2�Ļ������ֱ����ס��ҡ�������������û�ѧ��Ӧ���ʷֱ�Ϊ�ף�v(H2)��3 mol/(L��min)���ң�v(N2)��2 mol/(L��min)������v(NH3)��1 mol/(L��S)�������������кϳɰ��ķ�Ӧ����( )
A��v(��)��v(��)��v(��) B��v(��)��v(��)��v(��)
C��v(��)��v(��)��v(��) D��v(��)��v(��)��v(��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
þ�����Ԫ�����ڱ��о������⡰�Խ��ߡ�λ�ù�ϵ�����ǵ��������ƣ��������ǵĵ����ڹ���������ȼ��ʱ��ֻ������������������¶�﮵����ʵ�������ȷ����( )
A��Li2SO4������ˮ B��Li��Ũ����������ۻ�������
C��LiOH��ˮ��Һ����ʹ��̪��� D��Li2CO3���ȷֽ⣬����Li2O��CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�л�������A����Է���������ΧΪ100~130����������֪������̼�������������֮��Ϊ46.66%������Ϊ������û������������ຬ̼��˫���ĸ���Ϊ�� ��
A��1 B��2 C��3 D��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ɽ��ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ж�����ת������ʶ�У�����ȷ���ǣ� ��
A�����ˮ��������������ʱ��������Ҫת��Ϊ��ѧ��
B����������ʱ��������Ҫת��Ϊ����
C��úȼ��ʱ����ѧ����Ҫת��Ϊ����
D���׳�ƹ���ʱ������ȫ��ת��Ϊ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com